The first forays into lung cancer screening began in the 1970s, with two trials evaluating the use of chest x-ray and sputum cytology to detect disease. Those trials showed no reductions in mortality, advocating against the practice of lung cancer screening.
In the 50 years since that time, advances in imaging, research, and clinical trials have built a strong case for the benefits of lung cancer screening. The introduction of low-dose computed tomography (LDCT) and evidence that use of this tool in high-risk populations significantly reduces lung cancer mortality, as shown in the US National Lung Screening Trial (NLST) and the NELSON trial, validate the importance of lung cancer screening in selected individuals.
As a result, the United States adopted LDCT-based screening of high-risk individuals in 2013 in accord with US Preventive Services Task Force (USPSTF) guidance. Similarly, implementation of the NHS England Targeted Lung Health Checks program is currently underway in the United Kingdom following the success of the UK Lung Cancer Screening trial, despite some hiccups imposed by COVID-19.
As John K Field, PhD, FRCPath, of the University of Liverpool in the United Kingdom, detailed in his talk at the 2021 IASLC CT Screening Symposium, “Lessons Learnt from LC Screening Studies Worldwide Towards Optimal Implementation,” a number of factors need to be considered when executing national lung cancer screening programs to ensure their manageability and success. These include who, when, and how to screen; the required workup for suspicious nodules; how to treat suspicious nodules; how often to repeat screening; how long to continue screening; and how much (Fig. 1).
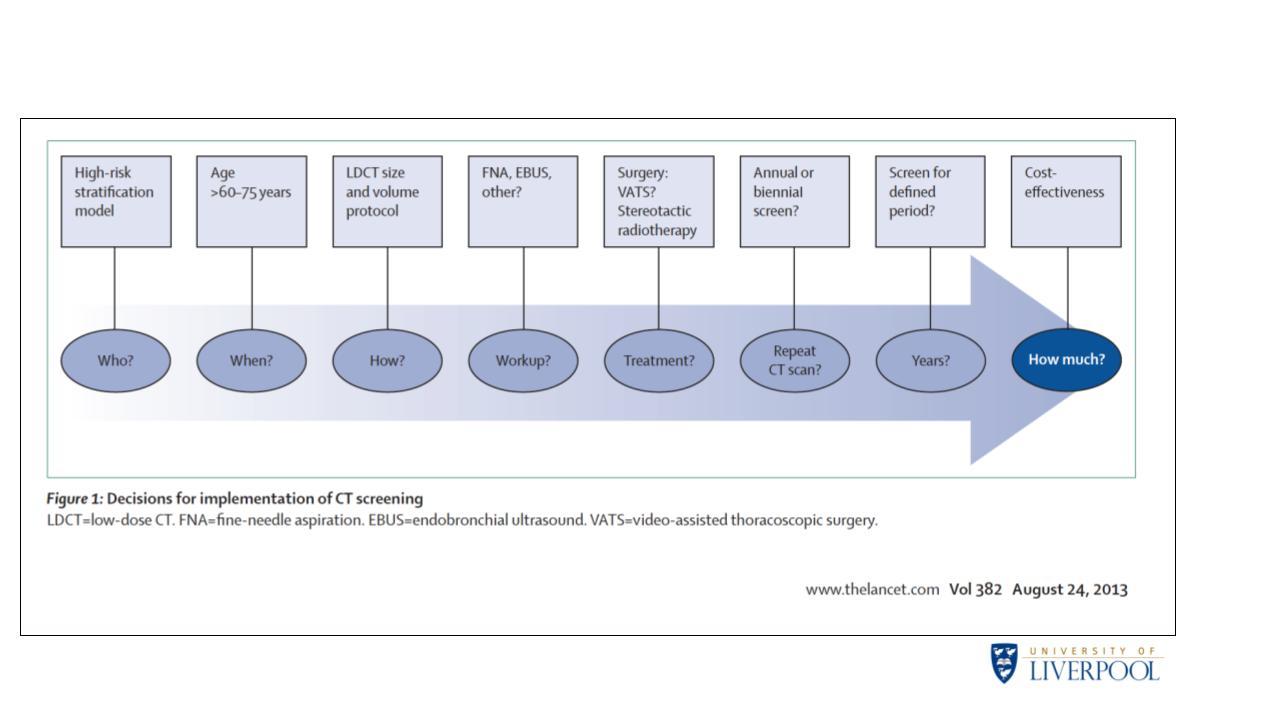
While there is little ambiguity in how to address some of these issues—for example, surgery remains the preferred treatment for CT-detected nodules—the best strategies for other issues remain less clear. Dr. Field drew from a wealth of clinical data for insight into how to tackle some of the more ambiguous issues that pertain to lung cancer screening programs.
Identifying High-Risk Individuals
In terms of who to screen, previously only age and smoking history were used to identify high-risk individuals for screening, and this remains true in the United States, despite recent updates to the USPSTF screening guidelines.1
Lung cancer screening programs vary slightly based on the age ranges deemed suitable for screening. For example, the USPSTF recommends screening high-risk individuals aged 50 to 80 years, whereas general consensus in Europe includes high-risk individuals aged 55 to 75 years.
However, beyond age, the NHS England Targeted Lung Health Checks program utilizes risk models from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial2
and the Liverpool Lung Project (LLP)3
to identify individuals suitable for screening. Prof. Field says these models “have demonstrated on countless occasions the power of risk-prediction modeling.” The USPSTF screening guidelines do not utilize any risk-prediction models, which some view as a major shortcoming of the recent updates.1
,4
Sex Differences
Another consideration regarding who to screen pertains to sex. Data from the NLST, NELSON, and LUSI trials suggest that women may derive greater benefit from lung cancer screening as compared with men.5
“This particular piece of work needs much greater attention in the future in implementation studies to confirm if this is true,” Prof. Field remarked.
Promoting Recruitment and Adherence
Prof. Field noted that more attention is now paid to informing all potential participants about the harms and benefits of lung-cancer screening. “Medicare and Medicaid Services have even included a payment within their funded CT-screening programs for shared decision-making,” he remarked.
Still, research suggests that certain communities are not engaged in lung-cancer screening, requiring extra effort to identify and convince these individuals of the benefits of screening. “This naturally requires a great deal of further work,” Prof. Field said.
Assessment of Nodule Size
With regard to how to screen, historically nodule size was routinely based on measuring the longest diameter of a lesion. However, nodule volume estimation using 3D reconstruction of thin CT slices has proven to be a more accurate and reproducible measure of nodule size given imperfections in the shape of lesions.6
Several European lung cancer screening trials incorporated volumetry in the study designs, prompting a European position statement on lung cancer screening that recommends this particular approach for lesion assessment.7
Prof. Field noted that in 2021 an update of the Lung Imaging Reporting and Data System (Lung-RADS) screening guidelines introduced volumetry assessment as a more reproducible alternative to manual linear measurements,2
leading to better alignment on “both sides of the pond.”
Screening Frequency
“The debate around annual or biennial screening remains undecided, with some arguing that individuals who have a clear baseline scan should potentially be considered for biennial screening, but re-evaluated during the life of their screening cycle,” Prof. Field stated.
For individuals with detectable lesions, volume doubling time can be used as an imaging biomarker to classify nodules according to their risk for growth, in turn guiding the necessary frequency of follow-up scans. Prof. Field also noted that blood biomarkers hold the potential to help tailor screening intervals for each individual.
Prof. Field did point out that the 2021 USPSTF lung cancer screening updates included “a very practical and worthwhile addition: the discontinuation of annual screening once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery.”1
Cost-Effectiveness
“The success of lung cancer screening will depend on cost-effectiveness,” Prof. Field emphasized. He showed data illustrating that several modeling analyses based on data from the United States, Canada, the United Kingdom, and Switzerland support the cost-effectiveness of LDCT-based lung cancer screening when applying country-specific thresholds for willingness to pay.5
Prof. Field also believes that LDCT-based screening can be made even more cost-effective if smoking-cessation strategies are applied in tandem. “It’s recognized that integrated smoking cessation needs to run alongside CT screening,” he emphasized.
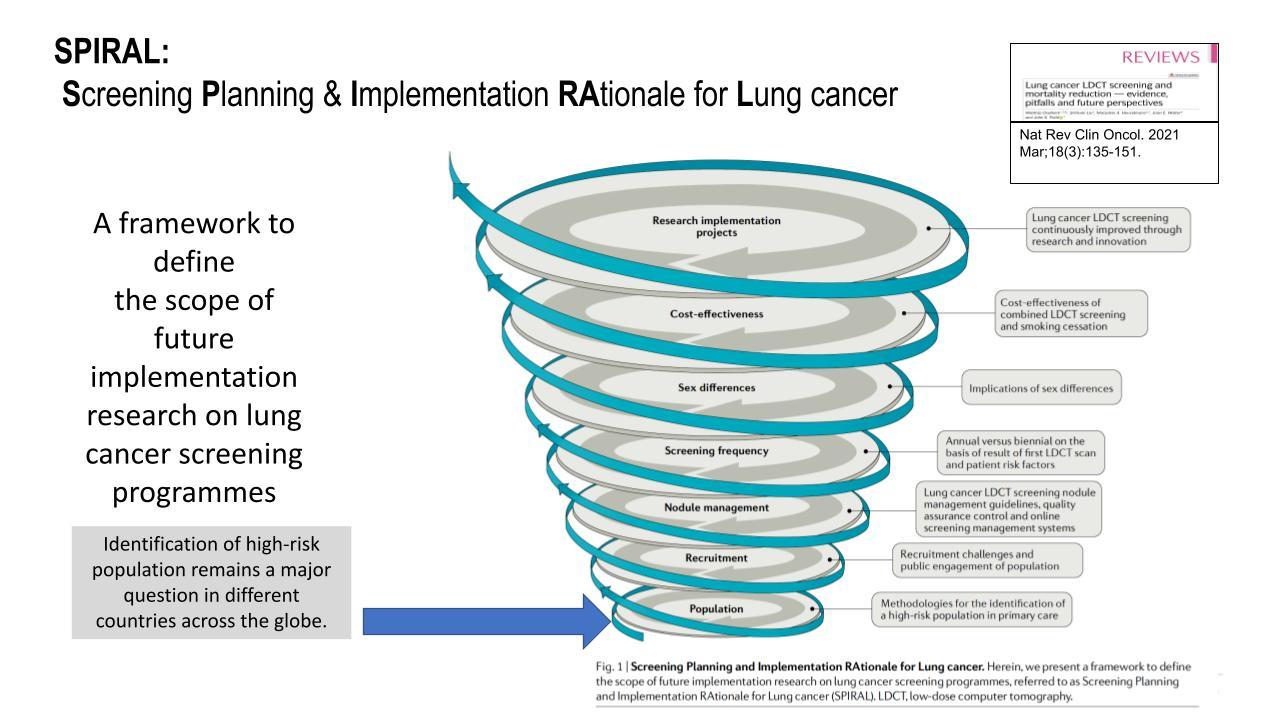
Future Implementation Research
Prof. Field provides the “SPIRAL’ framework (Fig. 2) to define the scope of future for lung cancer screening implementation research, (Screening Planning and Implementation Rational for lung cancer) in the Nature Reviews in Clinical Oncology 2020 publication.5
- 1. a. b. c. US Preventive Services Task Force. Lung cancer: Screening. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-ca…. Last updated March 9, 2021. Accessed May 2, 2021.
- 2. a. b. Tammemagi CM, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, et al. Lung cancer risk prediction: prostate, lung, colorectal and ovarian cancer screening trial models and validation. J Natl Cancer Inst. 2011;103(13):1058-68.
- 3. Field JK, Vulkan D, Davies MPA, Duffy SW, Gabe R. Liverpool Lung Project lung cancer risk stratification model: calibration and prospective validation. Thorax. 2021;76:161-8
- 4. Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US Preventive Services Task Force. JAMA. 2021;325(10):988-997.
- 5. a. b. c. Oudkerk M, Liu S, Heuvelmans MA, et al. Lung cancer LDCT screening and mortality reduction—evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18:135-151.
- 6. Heuvelmans MA, Walter JE, Vliegenthart R, et al. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax. 2018;73(8):779-781.
- 7. Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18(12):e754-e766.






