Although the initial research into adoptive T-cell therapy for solid tumors was related to tumor-infiltrating lymphocytes, there is a growing interest in the use of adoptive T-cell therapy for the treatment of these diseases.
As part of Friday’s Plenary session “PL03: Bench to Bedside (Immunology),” Prasad S. Adusumilli, MD, FACS, attending and deputy chief of thoracic surgery at Memorial Sloan Kettering Cancer Center, gave an update on the use of cell therapies in solid tumors, specifically thoracic cancers.
According to Dr. Adusumilli, who is also a current member of the IASLC Mesothelioma Committee, and an IASLC fellow 2008-2010, there is increasing research into harvesting patients’ peripheral blood lymphocytes in the blood bank and transducing them with a synthetic receptor (chimeric antigen receptor [CAR] or T-cell receptor [TCR]), expanding them at cell manufacturing facilities, and giving them back to the patient following lymphodepletion.
There are approximately 84 clinical trials in progress looking at TCR T-cell therapy for solid tumors; most of these are targeting melanoma. Similarly, there are many CAR T-cell trials being translated to phase II trials in solid tumors.
In his lab, Dr. Adusumilli said his team is focusing on CAR T-cell therapy for thoracic cancers.
“We all know of the CAR T-cell successes in hematologic malignancies,” he said. “The challenge in the next chapter is to make it successful in solid tumors.”
During his presentation, Dr. Adusumilli focused on several aspects of researching CAR T-cell therapy in thoracic cancers.
The first necessary step, he said, is to select a target antigen.
“This antigen should be expressed or overexpressed in the tumor cells, with limited expression in normal cells,” Dr. Adusumilli said.
In his lab, he and his colleagues have chosen to target mesothelin, a cancer-associated cell surface antigen. The expression of mesothelin has been shown to be associated with cancer aggressiveness. “If an antigen expression on cancer is associated with aggressiveness, it is unlikely that the cancer cell will shed the antigen and develop immune escape,” Dr. Adusumilli said.
Next, they worked to design a single-chain variable fragment (scFV) to successfully recognize and bind to the mesothelioma tumor cell.
“We have chosen a scFV in a way that it targets the cancers cells with high mesothelin expression and spares the low mesothelin-expressing tissues,” he said.
New Approaches to Existing Challenges
In their research, Dr. Adusumilli and colleagues have also focused on the tumor immune microenvironment. They have systemically investigated large cohorts of thoracic tumors including lung adenocarcinoma, mesotheliomas, and lung squamous cell carcinoma. In this research, they learned about the influence of balance between the effector and regulatory immune responses.
“Understanding tumor microenvironment is extremely important in order to translate adoptive T-cell therapy,” Dr. Adusumilli said.
Another important aspect of their research has been focused on the use of a regional delivery approach. Pleural mesothelioma encases the lung, heart, and pericardium. Because of this regional aggressiveness, Dr. Adusumilli and colleagues tested the use of a single dose of mesothelin-targeted CAR T cells to the pleural cavity as compared with a systemic approach. They found that this regional approach showed tumor eradication. Given regionally, the CAR T cells were immediately antigen activated, and they proliferated and expanded to higher numbers. In contrast, given systemically, the CAR T cells were sequestered in the lung and took time to reach the tumor, he said. During this time, the tumor can acquire resistance.
Dr. Adusumilli’s lab has also researched ways to overcome T-cell exhaustion. To investigate whether transferred CAR T cells get exhausted in the presence of overwhelming tumor burden, Dr. Adusumilli and colleagues used a mouse model with large tumor burden to administer a low dose of CAR T cells.
“Initially, the tumors regressed, but as CAR T cells were exhausted, the tumors relapsed,” Dr. Adusumilli said. “When we later gave anti–PD-1 antibody, this rescued the functionally exhausted CAR T cells.”
He noted that for patients with mesothelioma, a checkpoint blockade antibody is included in National Comprehensive Cancer Network guidelines as a second-line therapy. Therefore, patients on the mesothelin-targeting CAR T-cell clinical trials were able to receive PD-1 checkpoint blockade.
One clinical trial of mesothelin-targeted CAR T-cell therapy included approximately 41 patients with mesothelioma or metastatic lung and breast cancers who received intrapleural administration. A second trial included 11 patients with triple-negative breast cancer who received systemic administration. So far, there have been no cell production issues in these trials, Dr. Adusumilli noted. There has been no grade 3 or higher adverse events and no on-target or off-tumor toxicity.
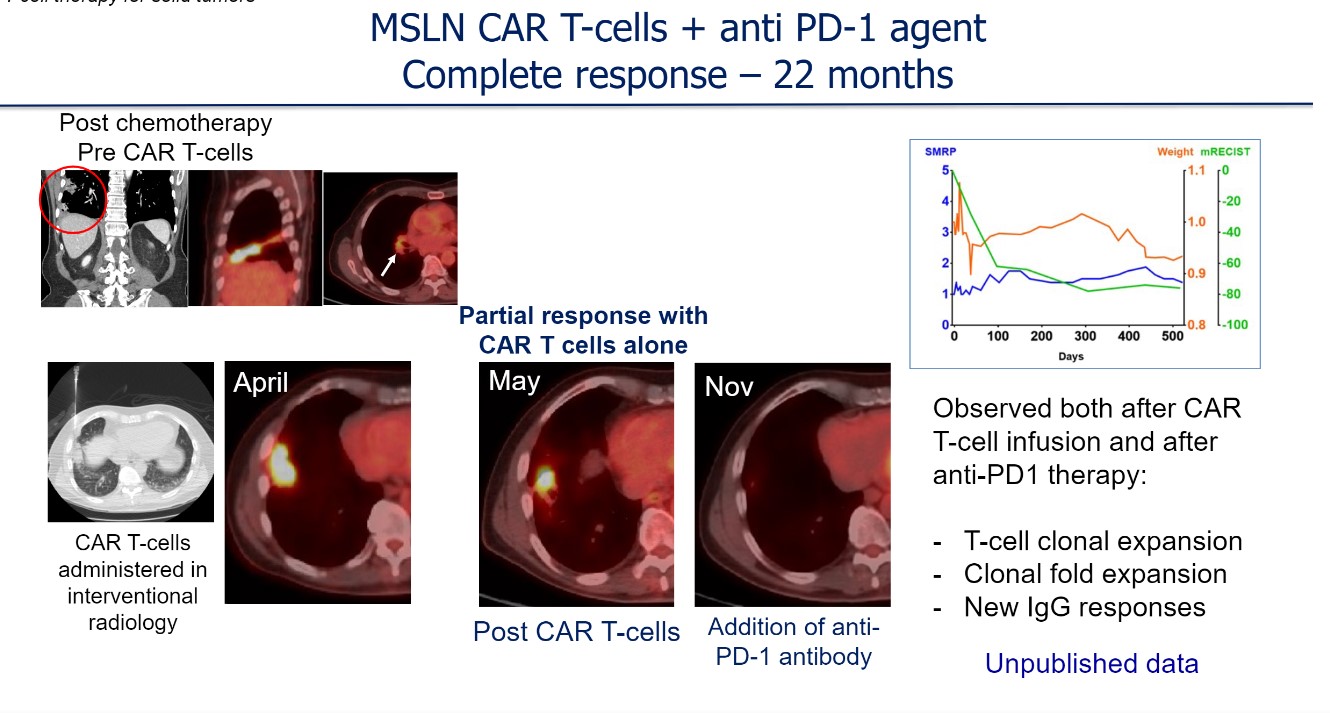
He discussed one example of a patient who received a single dose of CAR T-cell therapy. Approximately 1 month after infusion, there was tumor burden reduction (partial response). Shortly thereafter, with the addition of an anti–PD-1 antibody, the patient continued to experience a response and was able to remain without disease progression for more than 22 months (Figure).
Most importantly, based on the knowledge obtained in the above clinical trials and from ongoing preclincial research, Dr. Adusumilli and colleagues developed, obtained U.S. Food and Drug Administration Investigational New Drug approval, and initiated a novel clinical trial with a next-generation mesothelin-targeted CAR that is genetically engineered to encode a T-cell intrinsic checkpoint blockade strategy. Similar to previous trials, CAR T cells will be administered regionally in the pleural cavity in this ongoing trial.
This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 90 days at wclc2020.iaslc.org.