Oncologists currently have three main therapeutic strategies to choose from when managing patients with advanced NSCLC who lack targetable mutations: immunotherapy alone, immunotherapy-immunotherapy combinations, or immunotherapy-chemotherapy combinations. So how does one choose between them, and should we be selecting patients for therapy?
These questions were addressed during an Education Session entitled, “Pro-Con: Do We Need Biomarkers to Guide the Choice of Immunotherapy Treatment?”. While the first half of the session focused on whether oncologists should rely on biomarkers to select immunotherapy-based treatment for a patient, the second half focused on whether to use immunotherapy alone or immunotherapy plus chemotherapy and how biomarkers factor into that decision.
Should PD-L1, TMB, and Other Biomarkers Be Used to Guide the Type of Immunotherapy Combination Regimen?
“Biomarkers that predict response to immunotherapy are key,” stated Ticiana A. Leal, MD, of the University of Wisconsin Carbone Cancer Center, who took up the “pro” side of the argument for using biomarkers to guide treatment selection.
Dr. Leal focused her presentation on PD-L1 and tumor mutation burden (TMB), the two biomarkers that have been most rigorously assessed in clinical trials.
PD-L1 is the oldest predictive biomarker utilized in NSCLC practice to help guide immunotherapy decision-making. “PD-L1, even though it isn’t perfect, really is here to stay and can help us make clinical decisions regarding patient selection for treatment,” Dr. Leal stated.
Although numerous immunohistochemistry (IHC) assays are available for measuring PD-L1, the Blueprint PD-L1 IHC Assay Comparison Project showed very high concordance in measuring PD-L1 tumor cell expression for the Dako22C3, Dako 28-8, and Ventana SP263 assays, whereas the Ventana SP142 assay stained fewer tumor cells overall.1 All of the assays also demonstrated immune cell staining, but greater variability between them was observed.
“This project did show us that across these different assays, sometimes there can be misinterpretation of PD-L1,” stated Dr. Leal.
Despite these discrepancies, Dr. Leal showed data from several large phase III clinical trials supporting the value of using PD-L1 tumor expression as a biomarker to select patients for immunotherapy based on a higher likelihood of treatment benefit. Data from KEYNOTE-024, KEYNOTE-042, CheckMate 227, and IMpower110 collectively illustrate that high PD-L1 expression predicts for higher objective response rates (ORRs), longer progression-free survival (PFS), and better overall survival (OS) outcomes following treatment with anti-PD-1/PD-L1 monotherapy or combination therapy, compared with chemotherapy alone.2-6 Moreover, results from IMpower110 suggest that there is a similar OS improvement with use of single-agent immunotherapy versus chemotherapy regardless of whether the SP142, 22C3, or SP263 assays are used to select patients with high tumor PD-L1 expression.7
TMB, which indirectly assesses the neoantigen load of a tumor, also demonstrates good predictive value when measured in tumor tissue. Results of the CheckMate 026, CheckMate 227, and KEYNOTE-042 trials illustrate that high TMB correlates with higher ORRs and longer PFS; it has yet to be associated with an OS benefit.8-10 However, TMB selection cannot be applied to all regimens. Emerging data suggest that the predictive value of tissue-based TMB is abrogated when selecting patients for treatment with immunotherapy-chemotherapy combinations instead of single- or double-agent immunotherapy.11
Like tissue-based TMB testing, blood-based TMB assessment also shows promise, although this approach needs more study.7,12,13
Dr. Leal acknowledged that there is room to improve both PD-L1 and TMB as biomarkers. Toward this end, instead of relying solely on single biomarkers to select patients for immunotherapy, “I think potentially what we really need is a combination of biomarkers,” Dr. Leal proposed.
Naiyer A. Rizvi, MD, of Columbia University Medical Center, next presented the “con” side of the argument. “I’m presenting on the thesis that immune checkpoint blockade is beneficial for all lung cancer patients, regardless of PD-L1, TMB, or other biomarkers,” he stated.
Dr. Rizvi’s position is due, in part, to the fact that the cutpoints used to select patients for treatment are not well defined. For example, in CheckMate 227, patients with PD-L1 expression > 1% showed nearly identical outcomes as patients with PD-L1 expression < 1% following treatment with nivolumab plus ipilimumab.7 In KEYNOTE-024, where selection of patients with ≥ 50% PD-L1 expression was useful for predicting long-term survival,3 Dr. Rizvi argued that it still remained a suboptimal biomarker.
“Even with this patient selection, 22% of patients had progressive disease as their best response. The 12-month progression-free survival was only 50%. Although PD-L1 is a useful indicator, I’m not sure that one would call it a totally robust biomarker,” Dr. Rizvi explained.
Other work has demonstrated that among patients with ≥ 50% PD-L1 tumor expression, the benefit of single-agent monotherapy largely occurs among patients with the highest levels of PD-L1 expression (eg, ≥ 90%).14,15
Other limitations in using PD-L1 as a biomarker stem from challenges in how it is measured. Results can vary depending on tumor heterogeneity, the interval between the time of the biopsy and treatment, analysis of primary versus metastatic lesions, and the criteria used to define PD-L1 positivity.16
Like PD-L1, TMB shows promise as a biomarker, but it is still far from ideal. “To understand why TMB is not a perfect biomarker, I think it’s important to understand what TMB represents. TMB is really a surrogate for neoantigens…. The challenge is that our ability to predict these neoantigens is very poor,” Dr. Rizvi explained.
The field lacks the skill to take whole-exome sequencing data and accurately determine the number of neoantigens produced, even with prediction algorithms.17 Other complicating factors exist as well. “For example, too much neoantigen heterogeneity arising from subclonal cell populations may actually be a bad thing by attenuating the response to immunotherapy.18 In addition, human leukocyte antigen class I loss of heterozygosity can occur, resulting in reduced neoantigen presentation and enhanced immune invasion.”.19 Dr. Rizvi indicated. He believes better appreciation of these different factors is necessary so that TMB can be refined to be a stronger biomarker.
The final point that Dr. Rizvi made for using immunotherapy without respect to biomarkers is that “TMB and PD-L1 are really only two components of the complex tumor genetic and immune microenvironment.” For this reason, Dr. Rizvi agreed with Dr. Leal that more biomarkers are needed to better guide immunotherapy decision-making.
“I think that there is a future for biomarkers in lung cancer,” he said. However, he caveated this by stating, “Biomarkers in the future that can be useful will require a complex understanding of genetic and immune microenvironment interplay.”
Immunotherapy Alone or Immunotherapy Plus Chemotherapy as Maintenance?
When it comes to long-term treatment of advanced NSCLC, Roy Herbst, MD, PhD, of the Yale Cancer Center in New Haven, Connecticut, asserted that immunotherapy alone should be the standard of care for patients with high PD-L1 expression.
“Remember how we only had chemotherapy back in the year 2000, with 1-year survivals of 33% or less? A paradigm shift was needed. Well, immunotherapy certainly has taken us by storm,” he stated.
As illustrated by the KEYNOTE-024 trial conducted in patients with a PD-L1 tumor proportion score ≥ 50%, immunotherapy alone is appropriate in the right population. Despite heavy patient crossover in the study, an updated data readout showed a 5-year OS rate of 31.9% among patients treated with pembrolizumab compared with an OS rate of 16.3% among patients treated with chemotherapy upfront.3 It is notable that this difference persisted between arms despite the fact that 66% of patients assigned to chemotherapy crossed over to receive pembrolizumab or another immunotherapy off study.
OS results of other trials, such as IMpower110, CheckMate 227, and EMPOWER-Lung 1, bolster those of KEYNOTE-024, demonstrating that single- or double-agent immunotherapy can confer much more durable OS benefits than those observed with chemotherapy in patients with higher levels of PD-L1 expression.6,9
“It’s really all about the tail of these curves,” Dr. Herbst emphasized, which flatten out, reflecting durable remission and perhaps hinting at possible cure.
“Can we cure metastatic NSCLC with immunotherapy? I think the answer is, yes. The KEYNOTE-24 data are historic,” Dr. Herbst asserted. “Do we need to personalize immunotherapy? Absolutely. We spent 20 years personalizing targeted therapy. It’s time to do the same for immunotherapy.”
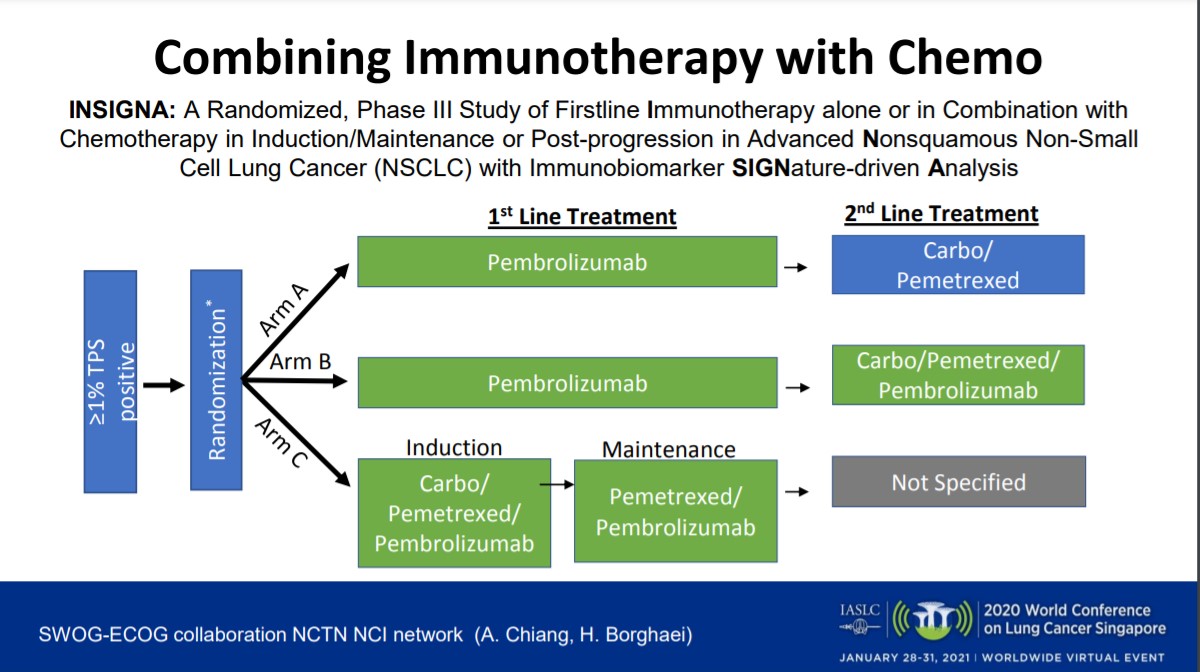
Dr. Herbst believes that the future of advanced NSCLC treatment centers on targeted immunotherapy. “It requires biomarkers and better combinations. We need more pioneer studies, studies with biomarkers, innovative trial designs, collaboration, and public-private partnerships,” he said. For example, this includes more trials like INSIGNA (NCT03793179), the National Lung Matrix Trial,20 the BATTLE trial,21 and the LUNG-MAP trial,22 which apply immunobiomarker-driven decision-making, new adaptive designs, and “master protocols.”
Marina Chiara Garassino, MD, of the Fondazione IRCCS Istituto Nationale dei Tumori in Milan, Italy, defended the opposite viewpoint. She presented a wealth of data supporting her position that combined treatment with immunotherapy and chemotherapy represents the best option for most patients with advanced NSCLC.
“I think that we need the chemotherapy, unfortunately,” Dr. Garassino stated.
Although Dr. Garassino agreed that the KEYNOTE-024 findings with pembrolizumab monotherapy are “clearly wonderful,” especially the updated 5-year OS data, “we have to remember that not all patients with PD-L1 ≥ 50% responded to the single agent. Only about 45% of these patients have shrinkage of the tumor with the single agent alone.”2,3
When examining the data more closely, subgroup analysis revealed that females and never-smokers did not respond well to single-agent pembrolizumab.3 Moreover, several clinical trials that compared immunotherapy—whether single-agent or double-agent immunotherapy—with chemotherapy showed crossing of the PFS and OS curves among the first 30% of patients, with chemotherapy initially beating out immunotherapy.4,5,8,23 Dr. Garassino argued that these data suggest that not all patients benefit from single-agent immunotherapy, which is the reason why immunotherapy-chemotherapy combinations are still necessary.
Chemotherapy is important given the complex tumor microenvironment that influences the response to immune checkpoint inhibitor treatment. “Chemotherapy is able to induce immunogenic cell death, and so it is able to enhance the activity of the T cells,” Dr. Garassino said. In addition, chemotherapy “is able to eliminate immunosuppressive components, such as the T regulatory cells, myeloid-derived suppressor cells, and macrophages with the M2 phenotype.”
The KEYNOTE-189 trial, which compared pembrolizumab plus pemetrexed/platinum followed by pembrolizumab-pemetrexed maintenance versus placebo plus pemetrexed/platinum followed by pemetrexed maintenance in PD-L1–unselected patients, clearly documents the benefit of an immunotherapy-chemotherapy combination. Median OS significantly improved with the addition of pembrolizumab to chemotherapy (22.0 vs 10.7 months; HR 0.56, 95% CI 0.45-0.70), and no early crossing of the curves was observed—the immunotherapy-chemotherapy regimen consistently remained on top.24
“If you ask yourself if it is better to start with chemotherapy and then use immunotherapy in the second part of the treatment, the answer is no,” Dr. Garassino stated. This is illustrated by the KEYNOTE-189 PFS2 data from patients who experienced disease progression on first-line treatment, which captured outcomes following a second-course of pembrolizumab in the pembrolizumab-chemotherapy arm or an initial course of pembrolizumab in the chemotherapy arm. The findings still favored upfront use of immunotherapy plus chemotherapy followed by immunotherapy rechallenge instead of chemotherapy followed immunotherapy (median PFS2: 17.0 vs 9.0 months; HR, 0.49, 95% CI 0.40-0.59).24
When determining which patients should receive chemotherapy, Dr. Garassino believes this decision should be made based on clinical characteristics—factors such as the aggressiveness of the tumor and whether patients are never-smokers—as opposed to disease histology or biomarkers like PD-L1 expression or TMB. In support of this approach, data from KEYNOTE-407, the companion trial to KEYNOTE-189, in patients with squamous histology, confirmed a significant OS benefit with pembrolizumab plus carboplatin/paclitaxel compared with carboplatin/paclitaxel alone.25 In addition, subgroup data from KEYNOTE-189, KN407,
CheckMate 9LA illustrate significant OS benefits with immuno-chemotherapy irrespective of PD-L1 expression levels.24,26
Despite ample data supporting the use of chemotherapy in combination with immunotherapy, there are still several unanswered questions about this treatment approach. “We don’t know how long to give the chemotherapy. We don’t know if there is a best chemotherapy regimen to be added,” Dr. Garassino said.
Dr. Garassino believes that until more mature data from trials like CheckMate 9LA are available to help answer some of these questions, particularly regarding the duration of chemotherapy, treatment decision-making will require an in-depth discussion with patients with advanced NSCLC to arrive at a shared decision regarding first-line treatment and maintenance.
References
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12(2):208-222.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. N Engl J Med. 2016;375(19):1823-1833.
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. KEYNOTE-024 5-year OS update: first-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50%. Ann Oncol. 2020;31(suppl 4):S1142-S1215.
- Mok TSK, Wu YL, Kudaba I, et al; KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830.
- Ramalingam SS, Ciuleanu TE, Pluzanski A, et al. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: three-year update from CheckMate 227 Part 1. J Clin Oncol. 2020;38 (suppl 15):9500.
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328-1339.
- Herbst RS, de Marinis F, Giaccoe G, et al. Clinical efficacy of atezolizumab (atezo) in biomarker subgroups by SP142, SP263 and 22C3 PD-L1 immunohistochemistry (IHC) assays and by blood tumour mutational burden (bTMB): Results from the IMpower110 study. ESMO Immuno-Oncology Congress 2019. Abstract 325.
- Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426.
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031.
- Herbst RS, Lopes G, Kowalski DM, et al. Association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Ann Oncol. 2019;30(suppl 5):v851-v934.
- Paz-Ares L, Langer CJ, Novello S, et al. Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Ann Oncol. 2019;30(suppl 5):v851-v934.
- Chen YT, Seeruttun SR, Wu XY, Wang ZX. Maximum somatic allele frequency in combination with blood-based tumor mutational burden to predict the efficacy of atezolizumab in advanced non-small cell lung cancer: a pooled analysis of the randomized POPLAR and OAK studies. Front Oncol. 2019;9:1432.
- Socinski M, Velcheti V, Mekhail T, et al. Final efficacy results from B-F1RST, a prospective Phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC). Ann Oncol. 2019;30(suppl 5):v851-v934.
- Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol. 2019;30(10):1653-1659.
- Sezer A, Kilickap S, Gümüş M, et al. EMPOWER-Lung 1: Phase III first-line (1L) cemiplimab monotherapy vs platinum-doublet chemotherapy (chemo) in advanced non-small cell lung cancer (NSCLC) with programmed cell death-ligand 1 (PD-L1) ≥50%. Ann Oncol. 2020;31(suppl 4):S1142-S1215.
- McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2(1):46-54.
- Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572-576.
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463-1469.
- Montesion M, Murugesan K, Jin DX, et al. Somatic HLA class I loss is a widespread mechanism of immune evasion which refines the use of tumor mutational burden as a biomarker of checkpoint inhibitor response. Cancer Discov. 2020. doi: 10.1158/2159-8290.CD-20-0672.
- Middleton G, Fletcher P, Popat S, et al. The National Lung Matrix Trial of personalized therapy in lung cancer. Nature. 2020;583(7818):807-812.
- Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1(1):44-53.
- Herbst RS, Gandara DR, Hirsch FR, et al. Lung Master Protocol (Lung-MAP)-A Biomarker-Driven Protocol for Accelerating Development of Therapies for Squamous Cell Lung Cancer: SWOG S1400. Clin Cancer Res. 2015;21(7):1514-1524.
- Rizvi NA, Cho BC, Reinmuth N, et al; MYSTIC Investigators. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6(5):661-674.
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505-1517.
- Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657-1669.
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021:S1470-2045(20)30641-0.