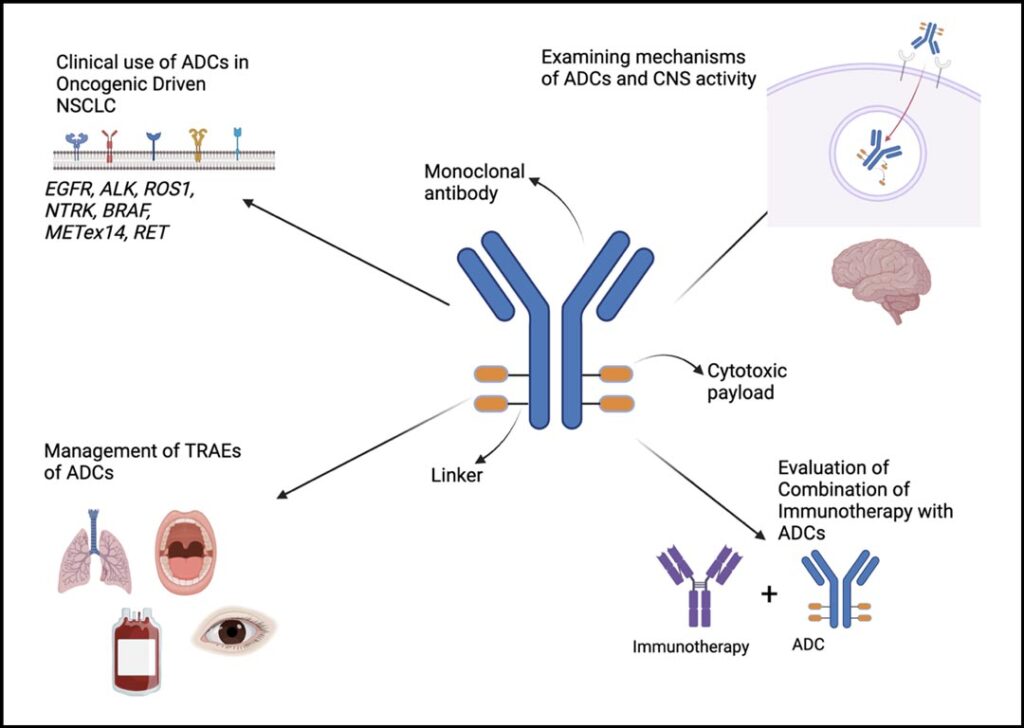
The management of non-small lung cancer (NSCLC) continues to undergo transformation with the introduction of personalized, targeted therapies for biomarker-defined patients as well as checkpoint inhibitors for patients with advanced and now earlier stage disease.

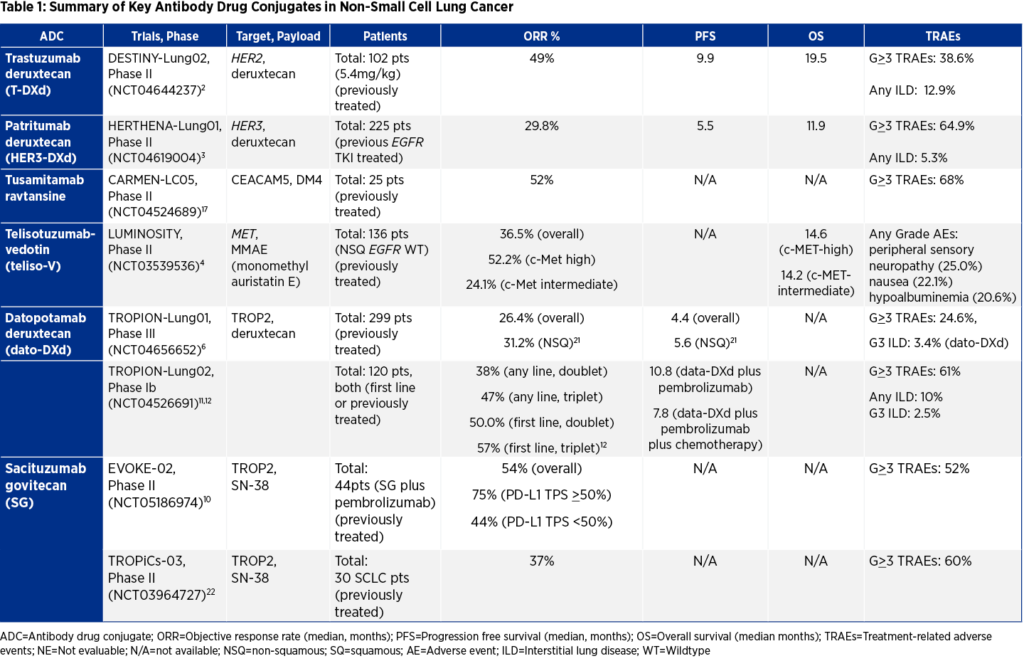
Recently, we have witnessed the dawn of a new revolution with antibody-drug conjugates (ADCs)—such as trastuzumab deruxtecan (T-DXd) for HER2 (ERBB2)-mutated, previously treated advanced NSCLC—which have had encouraging clinical results, including those from the phase II DESTINY-LUNG02 trial leading to T-DXd’s accelerated approval by the US Food and Drug Administration (FDA).1,2
As the field expands, other promising ADCs have emerged. Patritumab deruxtecan (HER3-DXd)3 targets HER3 in EGFR-mutated, previously treated patients with advanced NSCLC and has demonstrated meaningful clinical activity, including durable responses and central nervous system (CNS) activity in the phase II HERTHENA-Lung02 trial. Another encouraging ADC, telisotuzumab vedotin (teliso-V)4, a first-in-class c-Met protein-directed ADC, was granted a breakthrough therapy designation from the US Food and Drug Administration for patients with advanced/metastatic EGFR wild-type non-squamous (NSQ) NSCLC who have high levels of c-Met overexpression and were previously treated with platinum-based chemotherapy based on the phase II LUMINOSITY trial.5 Based on this data, the FDA has accepted a new drug application for dato-DXd for patients with advanced NSQ NSCLC. The agency is expected to evaluate the application this year.
An attractive target that has recently arisen is TROP2, a transmembrane glycoprotein that has been reported to be highly expressed in up to 60% to 75% of NSCLC. However, its role in cancer biology remains poorly understood. While two anti-Trop2 ADC contenders—datopotamab deruxtecan (dato-DXd) and sacituzumab govitecan—have demonstrated notable activity in early studies, where they will fit in the treatment paradigm remains unknown.

In patients with previously treated advanced NSCLC (second line and beyond), the highly anticipated read-out of the phase III TROPION-Lung01 trial showed improved progression free survival (PFS) of dato-DXd compared to docetaxel (4.4 months vs. 3.7 months) (p=0.004)6 and modest trends towards interim overall survival (OS) benefits of 12.4 months versus 11 months (HR 0.90) in the intention to treat (ITT) population. Interestingly, the PFS benefit witnessed was restricted to those patients with non-squamous (NSQ) histology (HR 0.63 and median 5.6 months) with a potential detrimental effect in patients with squamous histology (HR 1.38 and median 2.8 months). Of note, this OS data is not yet mature and further eagerly awaited with recent updates reconfirming clinical efficacy in the NSQ population in both PFS and OS.7
In addition to dato-DXd, sacituzumab govitecan was compared to docetaxel in a similar group of previously treated advanced NSCLC8 in the phase III EVOKE-01 trial. Although, this trial missed its primary endpoint of overall survival, there are reported trends in OS in the subgroup of patients who were previously refractory to anti-PD-(L)1 agents.9
TROP2-ADCs have also been examined in combination with immunotherapy in patients with treatment naïve, advanced NSCLC. Initial results of the phase II EVOKE-02 study that examined sacituzumab govitecan combined with pembrolizumab in two separate cohorts—PD-L1>50% and PDL1<50%—revealed promising objective response rates (ORR) of 75% and 44%, respectively.10 Similarly, the phase Ib TROPION-Lung02 assessed dato-DXd along with pembrolizumab plus or minus platinum chemotherapy (carboplatin or cisplatin) in multiple cohorts of PD-L1 unselected patients. Notable results were witnessed in the treatment naïve cohorts that received either doublet (pembrolizumab plus dato-DXd, ORR 50%) or triplet (pembrolizumab plus dato-DXd plus platinum chemotherapy, ORR 57%).11,12 Although these two trials exhibit promising results, further confirmatory phase III trials such as EVOKE-03,13 TROPION-Lung07,14 and TROPION-Lung0815 will help to further clarify the role of TROP2 ADCs in the first-line setting.

While dato-DXd and sacituzumab govitecan both target TROP2, the toxicity profile of these agents differs—with neutropenia and diarrhea being more common in the former and mucositis, eye toxicities, and pneumonitis in the latter—complicating our understanding of predicting ADC toxicities. It is worth pointing out that scant prior biological data, including the lack of biomarker identification, currently limit the ability for more scientifically focused studies of such agents. Therefore, it remains unclear whether anti-Trop2 agents can truly be viewed as “precision” therapies versus a repackaged way of administering standard chemotherapies.
Regarding the broadening utility of the TROP2 ADCs, it remains to be seen if the ongoing benefits will continue to play out in the final readouts and its potential frontline use. Future translational efforts as well as studies focusing on sequencing strategies will hopefully better define the role of these agents in NSCLC.
In addition to the outstanding issues related to TROP2 ADCs, other ADCs have not fulfilled their expectations, with sobering results. For example, tusamitamab ravtansine, which targets carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5), showed initial promising results in both the phase I/II trial as monotherapy16 and in the phase II CARMEN-LC05 trial17 in combination with first-line pembrolizumab with or without chemotherapy for advanced/metastatic NSQ NSCLC. However, after analyzing interim results, Sanofi recently announced that it will end its phase III CARMEN-LC03 trial that included previously treated advanced/metastatic NSQ NSCLC patients with high CEACAM5 expression (IHC >2+). Although there was an indication of a trend towards OS improvement, monotherapy tusamitamab ravtansine unfortunately failed to meet its dual primary endpoint of PFS compared to docetaxel and the trial has been discontinued.18
As the initial enthusiasm of ADCs has recently been tempered to some extent, several key outstanding issues need to be addressed if we hope to see this class of agents to reach wider impact (see Figure 1).
First is the challenge of biomarker selection and enrichment. Currently, the only predictive biomarker for this class of drugs has been HER2 alterations, specifically for patients treated with trastuzumab deruxtecan, thus highlighting the significant need for investing in more translational efforts. These efforts will become increasingly important as we begin to move ADCS in the frontline and curative setting. Another question is the appropriate dosing, especially in combination therapies with chemoimmunotherapy regimens.
As we continue to learn about the mechanisms of action, specifically target engagement and internalization, how we optimize both payloads and drug antibody ratios will be a key factor as we broaden their use to more patients. Furthermore, it will be of the utmost importance to consider the balance of the treatment adverse events—especially with interstitial lung disease, cytopenia, mucositis, and other side effects in common with chemotherapy—as ADCs become more widely used.
In addition, our gap in knowledge of understanding these mechanisms is highlighted by the recent results showing intracranial response rates for some of these agents. There is not yet a clear mechanism of how these bulkier forms of treatment are able to cross the blood brain barrier, underscoring the need for more research into this phenomenon
All in all, the future looks bright and encouraging for ADCs, and more importantly, our patients. Ongoing phase III trials—such as DESTINY-Lung04, HERTHENA-Lung02 and TeliMET NSCLC-01—not only hope to help further elucidate the optimal sequencing of ADCs, but also the continued examination of ADCs combined with immunotherapy plus chemotherapy as in TROPION-Lung04,19 TROPION-Lung07, 14 TROPION- Lung08,15 AVANZAR, and EVOKE03,13 to name a select few.
Lastly, additional developments of the dato-DXd program have included encouraging results in previously treated NSCLC patients with actionable oncogenic alterations in the phase II TROPION-Lung05 trial20 providing additional insights into ADC potential in patients with targetable alterations.
This unique class of therapeutics presents a tremendous opportunity to advance the field of NSCLC, but like any new class of drugs, these diamonds clearly need further refinement and polishing before we can nominate them as crown jewels in our ever-strengthening treatment armamentarium.
References
- 1. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for HER2-mutant non-small cell lung cancer. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-her2-mutant-non-small-cell-lung
- 2. Goto K, Goto Y, Kubo T, et al. Trastuzumab Deruxtecan in Patients With HER2-Mutant Metastatic Non-Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial. J Clin Oncol. Nov 1 2023;41(31):4852-4863. doi:10.1200/JCO.23.01361
- 3. Yu HA, Goto Y, Hayashi H, et al. HERTHENA-Lung01, a Phase II Trial of Patritumab Deruxtecan (HER3-DXd) in Epidermal Growth Factor Receptor-Mutated Non-Small-Cell Lung Cancer After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy. J Clin Oncol. Dec 10 2023;41(35):5363-5375. doi:10.1200/JCO.23.01476
- 4. Camidge DR, Bar J, Horinouchi H, et al. Telisotuzumab vedotin (Teliso-V) monotherapy in patients (pts) with previously treated c-Met–overexpressing (OE) advanced non-small cell lung cancer (NSCLC). Journal of Clinical Oncology. 2022;40(16_suppl):9016-9016. doi:10.1200/JCO.2022.40.16_suppl.9016
- 5. Press Release: AbbVie Announces Positive Topline Results from Phase 2 LUMINOSITY Trial Evaluating Telisotuzumab-Vedotin (Teliso-V) for Patients with Previously Treated Non-Small Cell Lung Cancer (NSCLC) https://news.abbvie.com/2023-11-29-AbbVie-Announces-Positive-Topline-Results-from-Phase-2-LUMINOSITY-Trial-Evaluating-Telisotuzumab-Vedotin-Teliso-V-for-Patients-with-Previously-Treated-Non-Small-Cell-Lung-Cancer-NSCLC
- 6. Ahn MJ, Lisberg A, Paz-Ares L, et al. LBA12 datopotamab deruxtecan (dato-DXd) vs. docetaxel in previously treated advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC): Results of the randomized phase III study TROPION-Lung01. Annals of Oncology. 2023;34:S1305-S1306. doi:10.1016/j.annonc.2023.10.061
- 7. Datopotamab deruxtecan (Dato-DXd) in patients with previously treated advanced non-small cell lung cancer (NSCLC): Nonsquamous (NSQ) histology in the phase III TROPION-Lung01 trial. https://www.esmoopen.com/article/S2059-7029(24)00406-X/fulltext
- 8. Garassino MC, Reznick D, Liu SY, et al. EVOKE-01: A phase 3 study of sacituzumab govitecan (SG) versus docetaxel in patients with non–small cell lung cancer (NSCLC) progressing on or after platinum-based chemotherapy and checkpoint inhibitors. Journal of Clinical Oncology. 2022;40(16_suppl):TPS9149-TPS9149. doi:10.1200/JCO.2022.40.16_suppl.TPS9149
- 9. Gilead Provides Update on Phase 3 EVOKE-01 Study. 1/22/24, 2024. https://www.gilead.com/news-and-press/press-room/press-releases/2024/1/gilead-provides-update-on-phase-3-evoke-01-study
- 10. Cho BC, Dols MC, Reyes Cabanillas R, et al. OA05.04 Sacituzumab Govitecan + Pembrolizumab in 1L Metastatic Non-Small Cell Lung Cancer: Preliminary Results of the EVOKE-02 Study. Journal of Thoracic Oncology. 2023;18(11)doi:10.1016/j.jtho.2023.09.041
- 11. Goto Y, Su W-C, Levy BP, et al. TROPION-Lung02: datopotamab deruxtecan (dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) in advanced non-small cell lung cancer (aNSCLC). Journal of Clinical Oncology. 2023;41(16_suppl):9004-9004. doi:10.1200/JCO.2023.41.16_suppl.9004
- 12. Press Release: Datopotamab deruxtecan combinations showed encouraging tumour responses in patients with advanced non-small cell lung cancer in TROPION-Lung02 Phase Ib trial. 6/5/2023, 2023. https://www.astrazeneca.com/media-centre/press-releases/2023/datopotamab-deruxtecan-combinations-showed-encouraging-tumour-responses-in-patients.html
- 13. Moskovitz M, Okamoto I, Chen P, et al. Abstract CT067: Pembrolizumab with and without sacituzumab govitecan as first-line treatment for metastatic non-small-cell lung cancer (NSCLC) with PD-L1 TPS ≥50%: phase 3 KEYNOTE-D46/EVOKE-03 study. Cancer Research. 2023;83(8_Supplement):CT067-CT067. doi:10.1158/1538-7445.Am2023-ct067
- 14. Okamoto I, Kuyama S, Girard N, et al. 1505TiP TROPION-Lung07: A phase III trial of datopotamab deruxtecan (dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) as first-line (1L) therapy in advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC) with PD-L1 expression. Annals of Oncology. 2023;34:S847-S848. doi:10.1016/j.annonc.2023.09.2536
- 15. Levy BP, Felip E, Reck M, et al. TROPION-Lung08: phase III study of datopotamab deruxtecan plus pembrolizumab as first-line therapy for advanced NSCLC. Future Oncol. Jul 2023;19(21):1461-1472. doi:10.2217/fon-2023-0230
- 16. Ricordel C, Barlesi F, Cousin S, et al. Safety and efficacy of tusamitamab ravtansine (SAR408701) in long-term treated patients with nonsquamous non–small cell lung cancer (NSQ NSCLC) expressing carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5). Journal of Clinical Oncology. 2022;40(16_suppl):9039-9039. doi:10.1200/JCO.2022.40.16_suppl.9039
- 17. Isambert N, Nagy T, Ravoire M, et al. Safety and efficacy of tusamitamab ravtansine in combination with pembrolizumab ± chemotherapy in patients with CEACAM5-positive nonsquamous NSCLC (CARMEN-LC05 phase II study). Journal of Thoracic Oncology; 2023:S46.
- 18. Press Release: Sanofi announces end of program evaluating tusamitamab ravtansine after a 2L NSCLC Phase 3 trial did not meet a primary endpoint. 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-21-06-30-00-2799759
- 19. Borghaei H, Waqar SN, Bruno DS, et al. TROPION-Lung04: Phase 1b, multicenter study of datopotamab deruxtecan (dato-DXd) in combination with immunotherapy ± carboplatin in advanced/metastatic non-small cell lung cancer (mNSCLC). Journal of Clinical Oncology. 2023;41(16_suppl):TPS3158-TPS3158. doi:10.1200/JCO.2023.41.16_suppl.TPS3158
- 20. Paz-Ares L, Ahn MJ, Lisberg AE, et al. 1314MO TROPION-Lung05: datopotamab deruxtecan (dato-DXd) in previously treated non-small cell lung cancer (NSCLC) with actionable genomic alterations (AGAs). Annals of Oncology. 2023;34:S755-S756. doi:10.1016/j.annonc.2023.09.2348
- 21. Press Release: Datopotamab deruxtecan improved progression-free survival vs. chemotherapy in patients with previously treated non-small cell lung cancer in TROPION-Lung01 Phase III trial. 10/23/23, 2023. https://www.astrazeneca.com/media-centre/press-releases/2023/datopotamab-deruxtecan-improved-progression-free-survival-vs-chemotherapy-in-tropion-lung01-phase-iii-trial.html
- 22. Ogita M, Kubo M, Kumamaru H. Prognosis of squamous cell carcinoma of the breast by biological subtypes and the efficacy of radiotherapy: The Japanese Breast Cancer Registry analysis. Journal of Clinical Oncology. 2023;41(16_suppl):561-561. doi:10.1200/JCO.2023.41.16_suppl.561