TERAVOLT: Global Registry Investigates Outcomes Among Patients With Thoracic Cancer and COVID-19
By Marina Chiara Garassino, MD, and Leora Horn, MD, MSc, FRCPC Posted: June 9, 2020 Marina Chiara Garassino Leora Horn The new SARS-CoV-2 (COVID-19) virus […] Read more
Lung Cancer Considered – Episode XXV – Mar 16, 2020 Dr. Marty Edelman Speaks With IASLC President Dr. Tetsuya Mitsudomi
Posted: April 16, 2020 In this latest episode of Lung Cancer Considered, the official podcast of the IASLC, Dr. Marty Edelman speaks with IASLC President Dr. Tetsuya Mitsudomi. Download the […] Read more
By Suresh Ramalingam, MD Posted: December 11, 2019 IN REFERENCE TO: Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced Non–Small-Cell Lung Cancer Treated […] Read more
JAVELIN Lung 200: Potential Reasons for Avelumab’s Failure to Provide OS Benefit
By Hossein Borghaei, DO, MS Posted: August 14, 2019 In 2015, the use of checkpoint inhibitors in the second-line setting for metastatic lung cancer became the standard of care with the […] Read more
c-MET Antibody–Drug Conjugates: Misconceptions Corrected, Excitement Explained— An Interview with Dr. Karen Kelly
Posted: April 1, 2019 The first in-human study evaluating telisotuzumab vedotin (Teliso-V)—a c-MET antibody–drug conjugate formerly known as ABBV-399— has shown encouraging activity for patients with c-MET–positive NSCLC.1 Teliso-V combines the […] Read more
An Interview with Dr. Federico Cappuzzo: Checkpoint Inhibitors Have Replaced Old Strategies
Posted: March 1, 2019 Federico Cappuzzo, MD, PhD, has been the director of Medical Oncology at AUSL della Romagna, Ravenna, Italy, since April 2016; in January 2017 he became the […] Read more
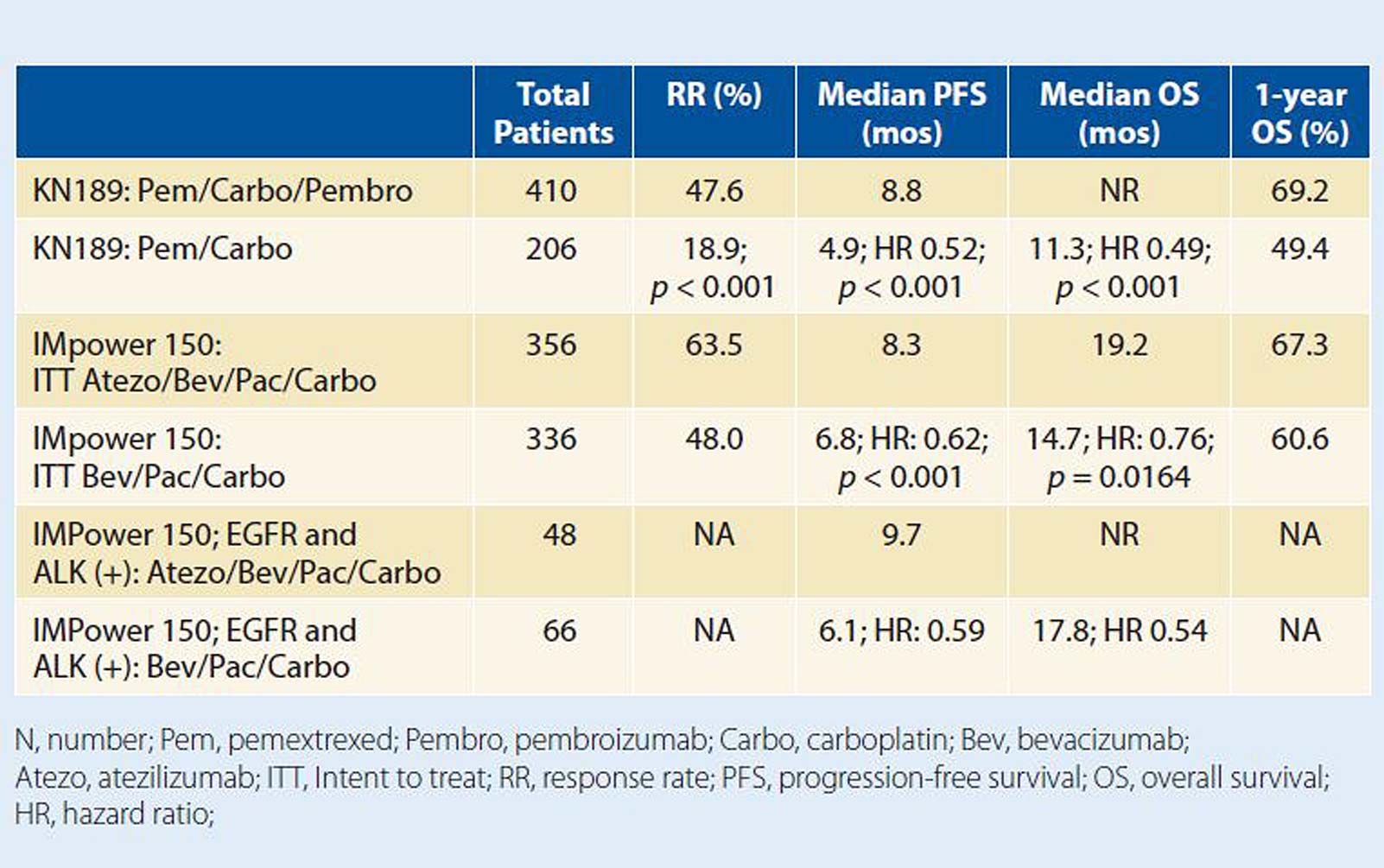
Posted: March 1, 2019 On December 6, 2018, the U.S. Food and Drug Administration (FDA) approved atezolizumab in combination with bevacizumab and chemotherapy, specifically paclitaxel and carboplatin, in advanced nonsquamous […] Read more
By Dr. Luis E. Raez, MD Posted: October 2018 Peru is one of the largest Latin American countries, with a population of approximately 28 million people. Cancer is not yet […] Read more
Dr. Howard 'Skip' Burris III Shares His Views on Clinical Trial Eligibility Criteria, Payer Involvement
Posted: June 2018 Howard A. “Skip” Burris III, MD, is the chief medical officer and president of clinical operations at Sarah Cannon, the Cancer Institute of HCA Healthcare, where he […] Read more
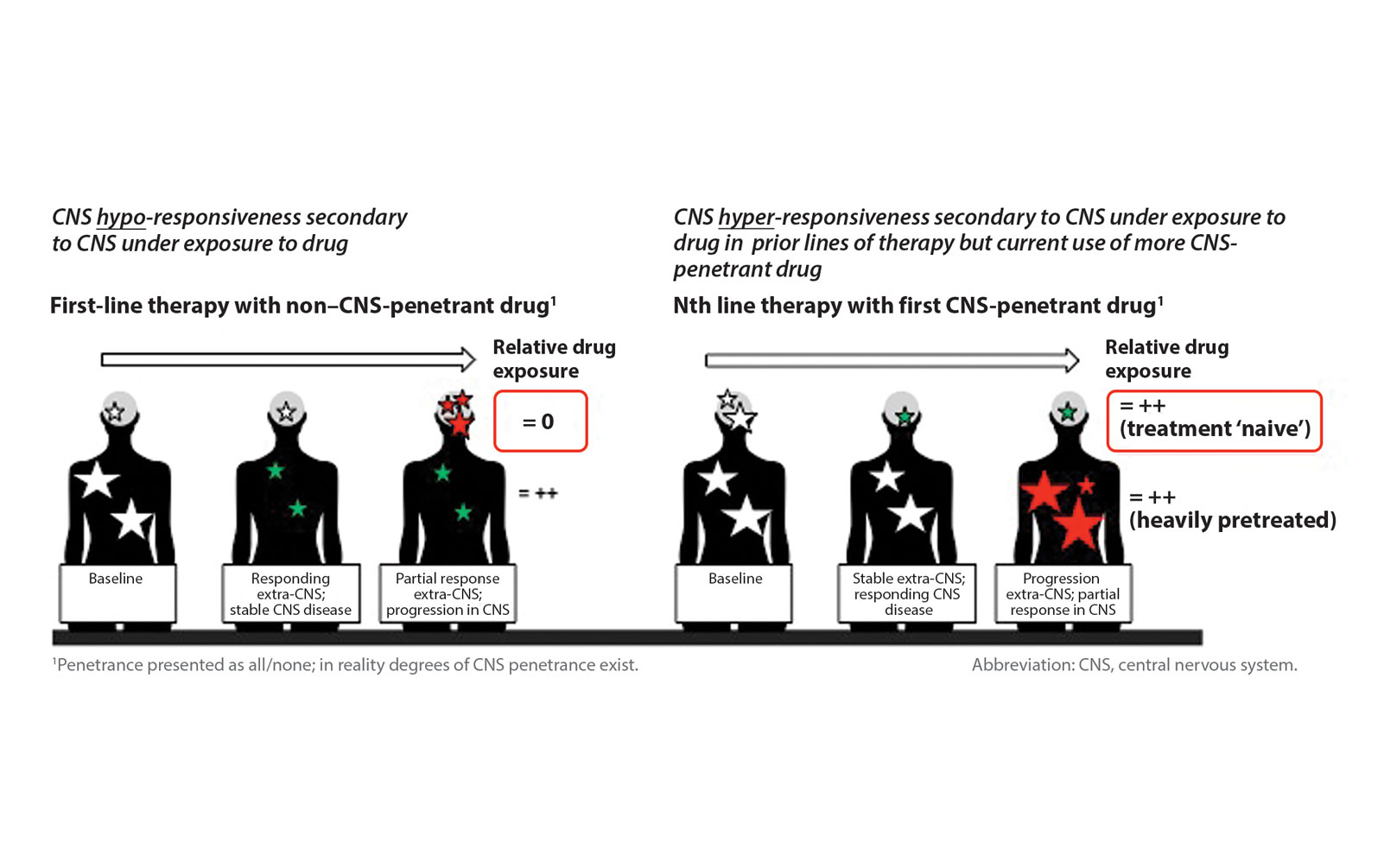
Posted: April 2018 In January 2018, the response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) working group—an international collaboration of cancer treatment, scientific, and imaging experts— published a new guideline on […] Read more