Data from multiple groups1,2 have shown the significant impact of COVID-19 infection among patients with malignancies, leading to high overall morbidity and mortality; this includes our own institution,3 which saw numerous cases of COVID-19 very early in the pandemic. Lung cancer stood out as the cancer (along with hematological malignancies) most significantly impacted by this disease. This is not surprising given the limited lung function, advanced age, and comorbidities common among patients with lung cancer.1,2,4 Consequently, there has been heightened interest in assessing the efficacy, safety, and overall benefit of COVID-19 vaccinations for patients with lung cancer. Several national and international societies have advocated for early immunization of patients with cancer, with one of the vaccines authorized by the U.S. Food and Drug Administration (FDA).4,5 Although all three vaccines authorized in the United States have undergone rigorous testing in randomized clinical trials, the exclusion criteria of these trials meant that most patients who were actively taking cancer-directed therapy could not participate, thereby limiting our knowledge of the safety and efficacy of these vaccines for people with cancer. Fortunately, over the last few months, significant and encouraging data have emerged in this regard.
Our group, as well as others, have assessed the development of COVID-19 immunity in patients with cancer in the United States, as measured primarily by serological tests such as the anti-spike IgG antibody assay—first among patients who survived COVID-19,6 then among cohorts of patients who have completed a series of authorized COVID-19 vaccinations (Pfizer-BioNTech, Moderna, or Johnson & Johnson).7 Our initial study on post-COVID immunity highlighted the robust immune response mounted by most patients with solid tumors, including lung cancers. It also helped to define vulnerable cohorts of patients, including those undergoing highly immune-suppressive therapies such as anti-CD20, CAR T-cell therapy, and stem cell transplantation, and those with hematological malignancies impacting the B-cell immune response (chronic lymphocytic leukemia, myeloma). These initial observations now appear fully translated to the post-vaccination experience. In our cohort of 200 patients from Montefiore Medical Center,7 as well as a cohort in a concurrently published international study,8 results replicated a very high seropositivity rate (98%) among many patients with solid tumors, including lung cancer, with titer levels matching those of control participants. Significantly lower seroconversion rates and titers were noted among the vulnerable cohorts identified above. Certain therapies, such as hormonal agents and immunotherapies, do not diminish the immune response at all; neither do they appear to create added toxicities. Although the numbers at present are still too small to support firm conclusions, follow-up cohorts suggest slight drop-offs in seroconversion among patients on active chemotherapy9—not a surprising finding. Nevertheless, positivity rates remain high enough to argue for vaccinations among patients on active chemotherapy.
While we await additional data that might provide further clarity on COVID-19 vaccinations for patients with lung cancer, our group applies available data and scientific rationale in considering these common questions:
- Should patients with a lung cancer diagnosis be vaccinated against COVID-19?
The answer is a resounding yes. COVID-19 can be devastating for a patient with lung cancer, whereas each of the vaccines appear to be exceedingly safe and very effective at preventing serious infections.
- Which vaccine should be given?
Our general recommendation has been for the patient to get whichever vaccine he or she has access to, as all appear to be very effective and safe for patients with lung cancer. That being said, the bulk of available data do show subtle differences favoring the efficacy of the mRNA vaccines. Longer term data are slowly emerging regarding waning immunity dependent on type and schedule of vaccinations, and this is an area we must continue to monitor. We recently published our study of 99 patients with cancer in which anti-spike IgG levels were checked in a follow-up after initial vaccination. The results indicate that whereas patients with lung cancer remained seropositive 4 to 6 months after initial vaccination (100% seropositivity in those with solid tumors), there was a significant decline in titers, especially with mRNA vaccines.10
- Are there any unique concerns about adverse events from COVID-19 vaccines for patients with lung cancer?
Although there is potential for safety concerns for patients receiving certain therapies, such as immune-modulating agents and checkpoint inhibitors, the available data thus far are highly reassuring with respect to safety. At this time, there is no contraindication for vaccinations in patients with lung cancer, regardless of treatment type.
- Should patients receive a booster shot?
Although the authorized series of vaccinations appear to be highly effective initially, some emerging data indicate waning immunity over time, leading to a spike in risk of breakthrough infections starting approximately 6 months after completed vaccinations. There are also concerns about variants, such as the now-dominant delta variant and any future variants that might emerge. For this reason, it was welcome news that the FDA and U.S. Centers for Disease Control and Prevention authorized booster vaccinations for the mRNA vaccines in patients who are immunocompromised—a broad category that includes a large cohort of patients with lung cancer on active therapy (with recent expansion to other populations as well). In conjunction with our study on waning immunity mentioned above, we also published our experience with 88 fully vaccinated patients with cancer who were tested for the presence of anti-spike IgG pre–booster vaccination and 4 weeks post–booster vaccination.10 The majority of patients received the Pfizer-BioNTech booster vaccination, although some received Moderna boosters. The very encouraging results showed that at 4 weeks post–booster vaccination, 79.5% of patients, including many with lung cancer, had antibody levels that were higher than after standard vaccination.
Thus, the questions are: Should patients with lung cancer receive a COVID-19 booster shot? When and with which vaccine? Is it better to stick with the same vaccine the patient already received, or is there a reason to switch?
Using the limited data we have thus far on booster vaccination,10,11 it appears safe and efficacious to recommend a booster dose to all patients with cancer who meet the current FDA and Centers for Disease Control and Prevention (CDC) criteria for booster vaccination. It also seems clear that in patients who had not seroconverted with the previous vaccine series, at least a certain percentage of those receiving the booster dose will seroconvert. Whether this seroconversion rate would be clinically meaningful is not yet known; however, such benefit is biologically reasonable to expect.
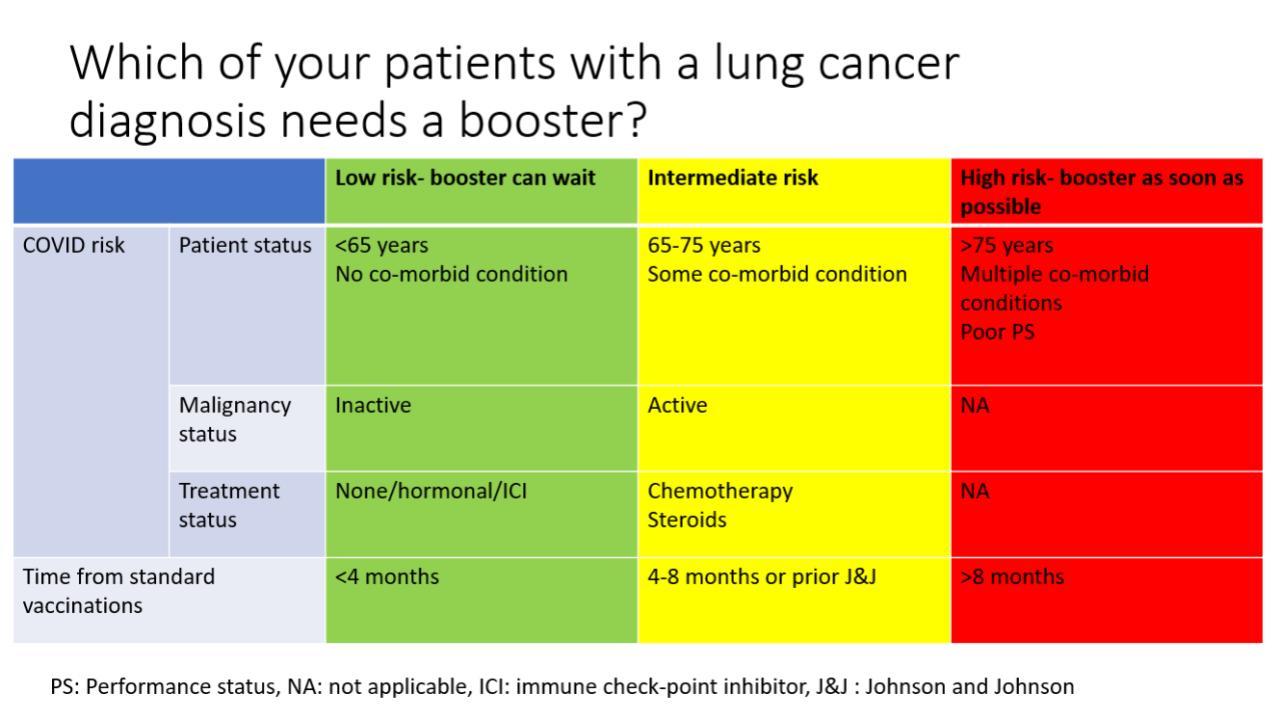
Until more data emerge as to the efficacy of COVID-19 booster shots specifically for patients with lung cancer, we take into consideration multiple individualized factors when deciding whether to proceed with a booster, including timing of the patient’s prior vaccination, the age of the patient, any comorbid conditions, and any ongoing therapy that might impact booster efficacy. For example, a frail, elderly patient with advanced lung cancer and severe chronic obstructive pulmonary disease who completed his or her vaccinations more than 6 months ago is an excellent candidate for a booster; even a breakthrough infection could result in potential significant morbidity, and this patient’s immunity from the prior vaccination could be waning at this point. A booster shot in such a case might be more urgent than in a stable, younger patient without comorbid illness who had lung surgery, received his or her vaccines 4 to 6 months ago, and is not on active therapy (Fig.).
Fig. Considerations for COVID-19 Booster Shots in Patients With Lung Cancer.
- Should we offer switch boosters?
The current guidance from the FDA and CDC is for patients to receive the same type of booster shot they had received previously; however, the FDA and CDC have also recently approved switch boosters for patients who may choose to receive a different type of booster shot from their previous shots. This topic, however, remains an area of active research and controversy. While scientifically this could make sense, especially in immune-suppressed patients who might not have responded to the prior vaccinations, we believe further clinical studies are needed to fully address this question. Our group, and others are pursuing such research avenues, hope to be able to address these key questions better.
- Is there laboratory testing that can assess COVID-19 immunity?
The answer is both yes and no. There are currently a multitude of tests that can provide data as to herd immunity on a community level; however, there is no indication that measured titers are meaningful for individual patients. One possible exception: for patients in the high-risk cohorts, completely negative antibody test results could highlight patient subgroups that need more intensive protective strategies, such as switch boosters and/or preventive antibody therapies. However, in general, such testing likely has limited value and cannot be recommended at present for the typical patient with solid tumors, such as patients with lung cancer.
In each of these areas of concern, as with so many other considerations during the COVID-19 pandemic, our focus should remain on the promotion of further research and rapid adaptation of emerging research as the pandemic unfolds. Science is key and should remain our governing principle.
- 1. a. b. Mehta V, Goel S, Kabarriti R, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10(7):935-941.
- 2. a. b. Kuderer NM, Choueiri TK, Shah DP, et al.; COVID-19 and Cancer Consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907-1918.
- 3. Desai A, Gainor JF, Hegde A, et al.; COVID19 and Cancer Clinical Trials Working Group. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18(5):313-319.
- 4. a. b. Garassino MC, Whisenant JG, Huang LC, et al.; TERAVOLT investigators. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914-922.
- 5. National Comprehensive Cancer Network. COVID-19 Resources. Accessed November 19, 2021. https://www.nccn.org/covid-19
- 6. Thakkar A, Pradhan K, Jindal S, et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer. 2021;2(4):392-399.
- 7. Thakkar A, Gonzalez-Lugo JD, Goradia N, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081-1090.e2.
- 8. Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 021;39(8):1091-1098.e2.
- 9. Naranbhai V, Pernat CA, Gavralidis A, et al. Immunogenicity and Reactogenicity of SARS-CoV-2 Vaccines in Patients With Cancer: The CANVAX Cohort Study. J Clin Oncol. 2021. [Epub ahead of print].
- 10. a. b. c. Shapiro LC, Thakkar A, Campbell ST, et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. [Epub ahead of print].
- 11. Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann Intern Med. 2021;174(9):1330-1332.






