Evaluation of mRNA expression of selected genes to tailor adjuvant chemotherapy did not improve survival outcomes in patients with resected stage II-III NSCLC, according to results of the ITACA study, presented during the Presidential Symposium.
“Despite its modest survival advantages cisplatin-based chemotherapy is considered the standard of care for early-stage completely resected non-small cell lung cancer,” said Silvia Novello, MD, PhD, of the department of oncology at the University of Turin, Italy. “There is still a need for identification of patients who will benefit from this approach.”
The phase III study enrolled 773 patients with completely resected stage II-III NSCLC from 31 centers in Italy, Germany, and Poland. Patients underwent genomic analysis soon after surgery and were randomly assigned to investigator’s choice of platinum-based doublet chemotherapy or to chemotherapy defined by molecular markers.
In the tailored treatment arm, tumors with high Excision Repair Cross Complementation 1 (ERCC1) and high thymidylate synthase (TS) were treated with single-agent paclitaxel (T1; 148 patients), tumors with high ERCC1 and low TS were treated with single-agent pemetrexed (T2; 43 patients), tumors with low ERCC1 and high TS were treated with cisplatin and gemcitabine (T3; 101 patients); and tumors with low ERCC1 and low TS, with cisplatin and pemetrexed (T4; 92 patients). The cut-off value for ERCC1 was 1.42 unitless ratio and was 0.50 unitless ratio for TS.
The primary endpoint was overall survival (OS), with recurrence-free survival and toxicity among the secondary endpoints. For the final analysis, all control arms were grouped and all tailored treatment arms were grouped. Patients and disease characteristics were balanced between the two arms. Adenocarcinoma was the most represented histology. In both arms, the median number of treatment cycles was four.
“Adjuvant chemotherapy customization based on the primary tumor tissue mRNA expression of ERCC1 and TS did not significantly improve overall survival and recurrence-free survival,” Dr. Novello said.
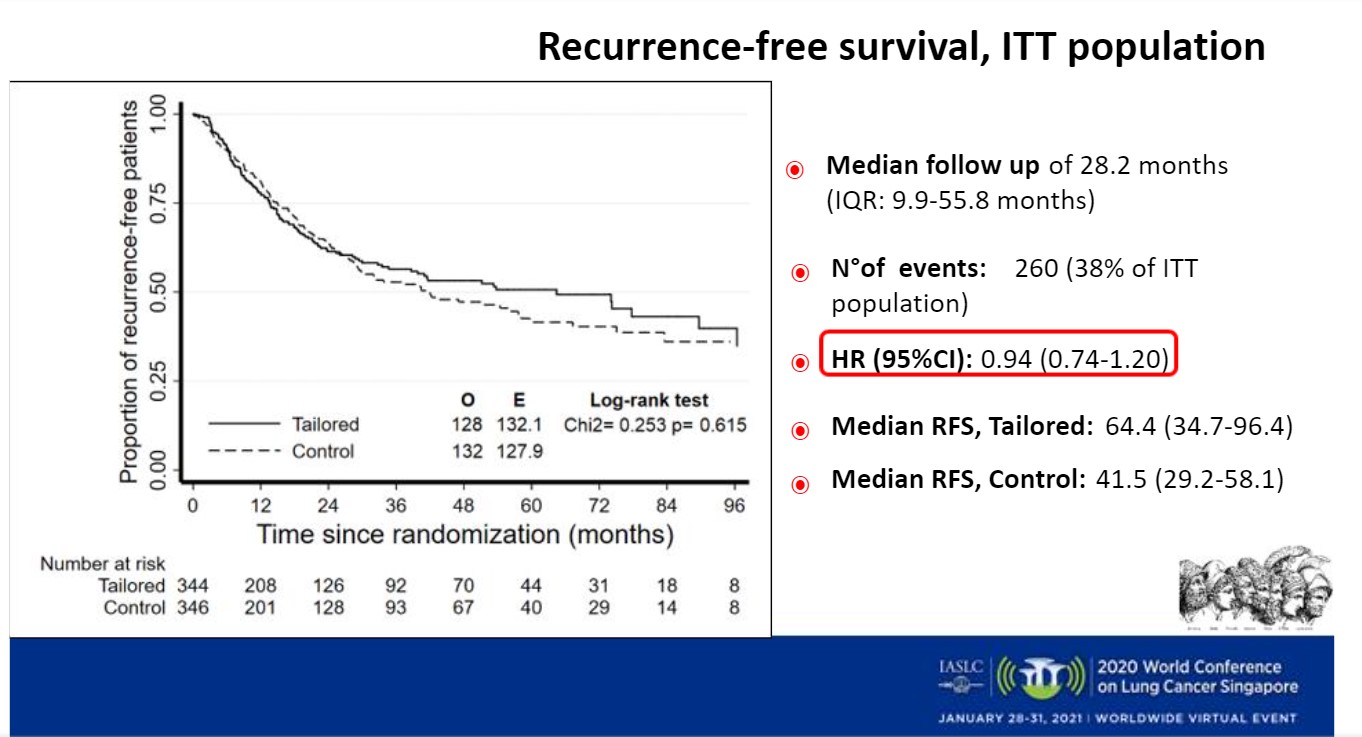
With a median follow-up of 28.2 months, there was no significant difference in OS between the tailored treatment arms and the control arms (HR = 0.76; 95% CI: 0.55-1.04). Median OS was 96.4 months for the tailored arm and 83.5 months in the control arm.
“No heterogeneity was detected between the different genomic profiles,” Dr. Novello said.
However, she noted a non-statistically significant trend for OS favoring the tailored treatment arm.
“When the final analysis was performed, the study was underpowered,” Dr. Novello said. “Only 46% of expected events were collected. Keeping the point estimate obtained and reaching those 336 [expected] events the interval confidence for the hazard ratio would be 0.61-0.94, therefore, significant.”
Results were similar for recurrence-free survival outcomes (Figure). With a median follow-up of 28.2 months, there was no significant differences between the tailored treatment and control arms (HR = 0.94; 95% CI: 0.74-1.20). Median recurrence-free survival was 64.4 months for the tailored treatment arm and 41.5 months for the control arm.
Results of subgroup analyses for OS and recurrence-free survival showed consistent results with the main analysis.
There was a reduction of toxicity favoring the tailored treatment arm, mainly regarding hematologic toxicity, Dr. Novello noted. This improvement was seen without compromising activity. The odds ratio of at least one grade 3-4 toxicity was 0.57 (95% CI: 0.42-0.78; p < 0.001).
“More comprehensive and high-throughput diagnostic techniques will be needed in order to tailor adjuvant chemotherapy–with or without immunotherapy,” Dr. Novello said.
A Deeper Look at ERCC1
Shinichi Toyooka, MD, PhD, of the department of general thoracic, breast, and endocrinological surgery at Okayama University, Japan, was the invited discussant for the ITACA trial.
“The purpose of this study was to demonstrate the usefulness of molecularly-based adjuvant chemotherapy for patients with resected stage II-IIIA NSCLC,” Dr. Toyooka said.
The first report demonstrating that the molecular marker ERCC1 may be useful for determining the regimen for adjuvant chemotherapy in advanced NSCLC was published in 2006. It demonstrated that patients with ERCC-1 negative tumors derived benefit from adjuvant chemotherapy, whereas, ERCC1-positive tumors did not.
In addition, TS expression is known to be inversely associated with pemetrexed response, Dr. Toyooka said.
Therefore, in this study, cisplatin was not used in the ERCC1 high arm and pemetrexed was used for patients with low TS expression. Unfortunately, the primary OS endpoint was not significantly improved with tailored treatment compared with the control arm, he said.
Dr. Toyooka discussed a report on the reliability of the molecular marker ERCC1 for stratification of cisplatin treatment. ERCC1 has four isoforms, he said, and only ERCC1-202 is a functional isoform that can repair platinum-DNA adducts. It has not been possible to develop ERCC1-202 specific primers for short lengths of RNA derived from paraffin formalin-fixed specimens, he said.
“In other words there is the possibility that functional ERCC1 is not accurately estimated in this kind of setting,” Dr. Toyooka said.
However, Dr. Novello noted that the treatment assignment for each arm assignment was guided by the results of the mRNA expression of ERCC1 and TS and not based on immunohistochemistry expression. The primers used for the study are detecting the entire ERCC1 gene and they were not designed to detect one specific isoform.
Dr. Toyooka also pointed out that the odds ratio of at least one grade 3/4 toxicity was significantly lower in the tailored group compared with the control group.
“Tailoring adjuvant chemotherapy based on ERCC1 and TS mRNA expression did not demonstrate a significant favorable overall survival I the present study,” Dr. Toyooka concluded. “Single-agent adjuvant chemotherapy based on molecular profile selection may be useful for reducing adverse events.”
In the future, using reliable molecular markers for selecting agents and patients is key for improvement of clinical outcomes of adjuvant therapy after radical surgery.