Whole-exome sequencing reveals that almost half of all patients with small cell lung cancer (SCLC) have a deleterious germline mutation which may be associated with the development of their disease (Abstract OA11.05). Moreover, these mutations could lend themselves to targeted therapy and screening in about 10% of individuals—a treatment approach for SCLC that has remained elusive.
“These findings suggest that SCLC may have an inherited predisposition, and this could have substantial implications for targeted therapy and directed cancer screening forSCLC patients and their families,” stated Nobuyuki Takahashi, MD, of the Center for Cancer Research (CCR) at the National Cancer Institute (NCI) in Bethesda, Maryland, who presented the research supporting these conclusions at the 2020 World Conference on Lung Cancer. The study was conducted by researchers including Camille Tlemsani, and Lorinc Pongor, and led by Anish Thomas, MBBS, MD of the Developmental Therapeutics Branch.
SCLC has continually lagged behind non-small cell lung cancer (NSCLC) in terms of advances in screening, treatment, and prognosis. Given the strong association between tobacco exposure and SCLC development, the inherited susceptibility to SCLC has long been overlooked as an important driver of disease. Moreover, researchers have been unable to identify molecular subtypes of SCLC to personalize therapy. “Targeted therapy hasn’t shown meaningful efficacy in multiple clinical trials,” leaving platinum-doublet therapy as the current standard of care for all patients with SCLC, according to Dr. Takahashi.
Dr. Takahashi and colleagues sought to address some these shortcomings by more closely examining the heritability of disease. They hypothesized that by characterizing inherited susceptible genetic alterations in SCLC, they could identify subpopulations of patients that may benefit from tailored targeted therapy.
Toward this end, the researchers performed whole-exome sequencing of germline DNA from peripheral blood mononuclear cells taken from 77 individuals with SCLC and 10 individuals with extrapulmonary small cell cancer. This discovery cohort consisted of individuals recruited across the United States for participation in NCI-sponsored clinical trials, all of whom had previously received platinum-based chemotherapy.
After sifting through 607 genes associated with the development of cancer, the researchers found that 43.7% of patients within the NCI cohort harbored a pathogenic or likely pathogenic mutation in 35 genes. In 9 patients (10.3% of the total cohort) the genetic variants identified were deemed to be actionable, offering up the opportunity for genetic counseling and screening for the patients and their family members.
Notably, two-thirds of the germline mutations identified occurred within genes involved in DNA damage repair pathways. Whole-exome sequencing of tumor tissue to capture somatic mutations revealed loss of heterozygosity of MLH1, BRCA2, and SMARCA4, emphasizing the importance of these genes in tumor formation and as potential treatment targets. .
When Dr. Takahashi’s group applied the same germline whole-exome sequencing process to an independent cohort of 79 patients with SCLC, they found that a similar proportion of individuals—40.2%—harbored a deleterious germline mutation, validating their initial results. Moreover, the types of mutations were also similar, with genes involved in DNA damage repair again predominating.
In another validation step, the group compared the sequencing results for the NCI cohort with those from a cohort of more than 53,000 cancer-free individuals from the Exome Aggregation Consortium (ExAC). Germline mutations within MUTYH, CHEK1, RAD51D, and BRCA2 were roughly 6 to 4,000 times more likely to be found in the NCI cohort with SCLC as compared with the cancer-free ExAC cohort.
In evaluating whether the pathogenic mutations were linked to clinical characteristics, the investigators found that germline genotype correlated with a higher likelihood of having a first-degree relative with a history of cancer (odds ratio [OR] 1.82) or lung cancer (OR 2.60).
The presence of pathogenic germline mutations was also independently associated with longer recurrence-free survival following platinum-based chemotherapy. Individuals with a germline genotype showed a 56% reduction in the risk of recurrence (HR 0.44; 95% CI 0.25-0.76) as compared with individuals lacking such mutations, even after adjusting for known prognostic factors including sex, age at diagnosis, and disease stage. However, these findings did not translate into an overall survival advantage (HR 0.92; 95% CI 0.55-1.54).
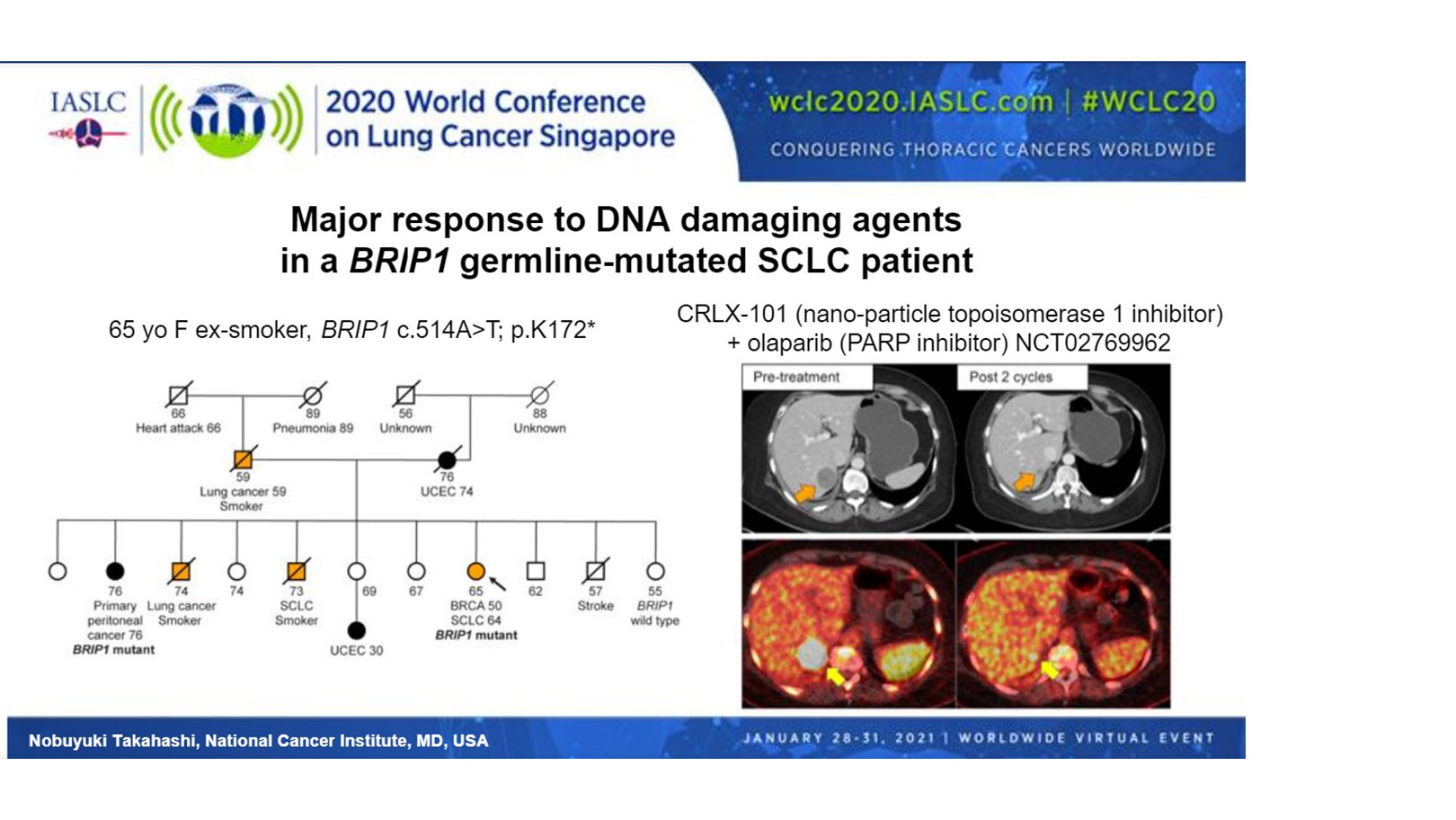
“The relevance of these observations for therapeutics was tested in a single patient with SCLC and a germline BRIP1 mutation. BRIP1 is involved in the BRCA1 complex, and its monoallelic pathogenic germline mutation is associated with a high risk of ovarian cancers,” said Dr. Takahashi. Indeed, the patient that Dr. Takahashi presented had multiple family members with endometrial cancer or lung cancer, and her sister had primary peritoneal cancer harboring the same BRIP1 variant (Figure).
Treatment of the patient with a topoisomerase 1 inhibitor, in combination with a PARP inhibitor, under their clinical trial (NCT02769962), resulted in a 64% reduction in the size of the patient’s liver lesion and resolution of cancer pain, according to Dr. Takahashi.
Stephen V. Liu, MD, of Georgetown University, who discussed Dr. Takahashi’s research, underscored the importance of these findings. “Progress is something we haven’t discussed in SCLC for quite some time. Platinum and etoposide have been our stagnant standard of care for many decades, well over 30 years,” up until very recently, he remarked.
Dr. Liu noted that although Dr. Takahashi’s work requires further validation, “when we look to the horizon, this can influence risk assessment and could lead to a way to personalized screening. Certainly it could influence treatment,” as illustrated by the outcomes achieved in the patient with the germline BRIP1 mutation.
He also challenged clinicians to keep an open mind with regard to SCLC etiology. “We’ve always associated small cell with tobacco use. Any family history of lung cancer is often attributed to a family history of smoking—a behavioral attribution and not necessarily a heritable risk. We may need to rethink that,” Dr. Liu remarked.
This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 90 days at wclc2020.iaslc.org.