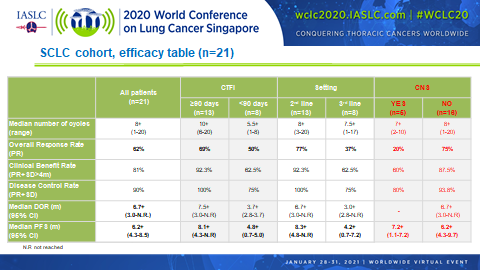
Combined treatment with lurbinectedin and irinotecan appears to pack quite a punch in patients with relapsed small cell lung cancer (SCLC) according to the results of a phase Ib/II trial (Abstract OA11.04). The overall response rate (ORR) among 21 patients who had received up to 2 prior lines of treatment reached 62% overall, with a median progression-free survival (PFS) duration of 6.2 months.
“The combination of lurbinectedin and irinotecan after failure of first-line therapy demonstrates good activity in SCLC. This has been observed in patients with platinum-sensitive and nonsensitive disease,” as well as in the third-line setting and in patients with brain metastases, said Santiago Ponce-Aix, MD, Head of the Lung Cancer Unit of the Medical Oncology Service of the 12 de Octubre University Hospital in Madrid, who presented the study findings.
As Dr. Ponce-Aix explained, SCLC is a transcription-addicted tumor. Lurbinectedin lends itself well to the treatment of SCLC because it selectively blocks oncogenic transcription by binding to DNA and modulating the activity of DNA binding proteins, including transcription factors. This leads to downregulation of several molecules in the tumor microenvironment, including IL-6, IL-8, CCL2, and VEGF, which in turn reduces tumor cell proliferation, suppresses activation of immune checkpoints, and inhibits angiogenesis.
In June 2020, the US Food and Drug Administration granted accelerated approval to lurbinectedin for the treatment of adult patients with metastatic SCLC with disease progression on or after platinum chemotherapy based on the results of a single-arm phase II basket study in which the ORR with single-agent lurbinectedin reached 35%.1
The current phase Ib/II trial (NCT02611024) was designed to build on the safety and efficacy of lurbinectedin monotherapy in line with preclinical research suggesting that adding irinotecan to lurbinectedin yields synergistic antitumor activity. Toward this end, patients with selected advanced solid tumors were enrolled into 1 of 3 cohorts exploring different doses and schedules of lurbinectedin and irinotecan.
The current analysis focused on patients with advanced SCLC who had received no more than 2 prior lines of chemotherapy and who were treated at the recommended doses of combination therapy—that is, lurbinectedin 2 mg/m2 on Day 1 in combination with irinotecan 75 mg/m2 on Days 1 and 8 every 3 weeks. Prophylactic G-CSF was also administered.
Of the 21 patients analyzed, all but 3 (86%) demonstrated some degree of tumor shrinkage with lurbinectedin plus irinotecan. These findings are encouraging considering that at baseline 81% of patients had extensive disease, 48% had liver metastases, 29% had bulky disease, and 24% had brain metastases.
In addition, promising ORRs and median PFS durations were observed regardless of whether patients were platinum sensitive (69% and 8.1 months, respectively) or platinum resistant (50% and 4.8 months, respectively), and regardless of whether lurbinectedin plus irinotecan was being delivered as second-line therapy (77% and 8.5 months, respectively) or third-line therapy (38% and 4.2 months, respectively).
Dr. Ponce-Aix described the toxicity of the lurbinectedin-irinotecan combination as “transient and manageable.” The most common grade 3/4 event was neutropenia, which occurred in 61.9% of patients. Thereafter, the most common grade 3/4 events were diarrhea (28.6%) and fatigue (23.8%), all of which were grade 3 in severity.
Despite the fact that 76.2% of patients experienced adverse events of grade ≥ 3 and 28.5% experienced serious adverse events, Dr. Ponce-Aix noted that there were “no discontinuations due to adverse events and no toxic deaths.” Still, 52.4% of patients required dose reductions, 28.6% required dose delays, and 33.3% required red blood cell transfusions.
Given the favorable findings, Dr. Ponce-Aix noted that the cohort of patients treated with up to 2 lines of prior therapy is being expanded to include 47 individuals to further explore the lurbinectedin-irinotecan combination in SCLC.
Patient Population Matters
Discussant Stephen V. Liu, MD, Director of Thoracic Oncology and Director of Developmental Therapeutics at the Lombardi Comprehensive Cancer Center of Georgetown University, indicated that the responses seen with lurbinectedin and irinotecan in patients with relapsed SCLC are impressive. However, he warned that “high response rates are something we have seen before in SCLC.” He recalled the experience with single-agent amrubicin, which showed a response rate of 67% in a phase II trial but that then fell to 31% when tested in a larger phase III trial.2,3
“It is not to say that lurbinectedin will behave like amrubicin,” Dr. Liu clarified. “The point is that in SCLC, a phase I or II study will enroll a highly selected patient population. For various reasons, perhaps chief among them survival bias, those patients will have much better outcomes than in a larger, arguably more representative phase III population. In larger studies, these numbers will fall.”
To ascertain whether the robust efficacy of lurbinectedin plus irinotecan holds steady and balances out the toxicity, Dr. Liu would like to see bigger studies of the combination in SCLC “to see if that juice is worth the squeeze.” It will also be important to determine whether the combination extends patient survival. “Although the response rates are nice… long survival is our goal and that remains elusive,” said Dr. Liu.
This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 60 days at wclc2020.iaslc.org.
References:
- Trigo J, Subbiah V, Besse B, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21(5):645-654.
- Inoue A, Sugawara S, Maemondo M, et al. Randomized phase II trial comparing amrubicin with re-challenge of platinum doublet in patients with sensitive-relapsed small-cell lung cancer: North Japan Lung Cancer Study Group trial 0702. Lung Cancer. 2015;89(1):61-65.
- von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32(35):4012-4019.