Because nearly one-half of patients with advanced cancer are eligible for immune checkpoint inhibitor (ICI)–based treatment, these therapies are now in widespread clinical use.1
ICIs—whether as monotherapy, in checkpoint inhibitor combinations, with chemotherapy, or after concurrent chemoradiation—are now incorporated into treatment regimens for most types of lung cancer, resulting in prolonged responses for a small proportion of patients.2
Despite extensive research over the past decade, predicting which patients are most likely to benefit from immunotherapy remains challenging. Predicting which patients are most likely to experience toxicities is even more difficult. Additionally, little is known about the administration of these agents to patients with relevant co-morbidities, such as pre-existing autoimmune disease or an organ transplant. Given the therapeutic mechanism, ICI use in these populations could hypothetically exacerbate the comorbidity or result in graft failure. Because these populations have been largely excluded from ICI clinical trials, real-world experience is critical to addressing these questions.
Autoimmune toxicities termed “immune-related adverse events” (irAE) distinguish ICIs from chemotherapy and molecularly targeted therapies. These events occur when ICI-induced immune activation reacts against host tissue. They may affect almost any organ, including the thyroid, lungs, liver, colon, skin, pituitary, heart, kidneys, joints, and muscles.3
,4
Further complicating clinical management, irAE may occur at almost any point during or even months after completing ICI treatment.5
Because of these toxicity profiles, ICI clinical trials have excluded not only patients with autoimmune disease or an organ transplant but also individuals receiving immunosuppressive drugs or who have chronic viral infections (such as hepatitis B or C) or interstitial lung disease. Indeed, it is estimated that strict eligibility criteria may exclude more than half of patients with lung cancer from ICI clinical trials.6
,7
To address this key knowledge gap, a small number of clinical trials have started to investigate ICI use in patients with such comorbidities (Table 1).
Table 1. Clinical Trials Investigating Immune Checkpoint Inhibitor Use in Patients With Comorbidities
| Comorbidity | Cancer Type | Immune Checkpoint Inhibitor | Clinicaltrials.gov Identifier |
|---|---|---|---|
| Autoimmune Disease | |||
|
Cohort 1: RA, psoriasis, GCA/PR, SLE Cohort 2a: UC, Crohn’s, MS |
NSCLC | Nivolumab | 03656627 |
| Any autoimmune disease | Advanced Cancers | Nivolumab, pembrolizumab, or ipilimumab | 03140137b |
| Chronic Viral Infection | |||
| HIV, HBV, HCV | NSCLC | Durvalumab | 04499053 |
| HIV | Solid Tumors | Nivolumab | 04514484 |
| Poor Performance Status | |||
| ECOG 2 | NSCLC | Pembrolizumab | 04253964 |
| ECOG 2 | NSCLC | Pembrolizumab | 04297605 |
| ECOG 2 | NSCLC | Pembrolizumab | 02733159 |
| ECOG 2 to 3 | NSCLC | Durvalumab | 04108026 |
Also supplementing published ICI trials are observational studies describing real-world clinical experiences with ICI therapy. In some instances, findings differ substantially. For example, phase III anti–PD-1/–PD-L1 monotherapy trials8
report rates of pneumonitis generally ranging from 3% to 5%. However, institutional series8 describe incidence rates closer to 20%. It is unclear if this discrepancy reflects differing patient characteristics or increased recognition and diagnosis of irAE.9
Despite the limitations of such retrospective studies, they do provide some insights into ICI administration in patients with autoimmune disease, end-organ dysfunction, organ transplantation, and other comorbidities.
Autoimmune Disease
Defining and diagnosing autoimmune diseases is often quite challenging. Whereas a diagnosis of cancer often requires only assessment of tissue biopsy, the diagnosis of an autoimmune disease may incorporate clinical features, physical examination findings, serologies, radiographic studies, and histologic examination. Additionally, characteristic laboratory findings of certain autoimmune diseases, such as antinuclear antibodies, may be present in more than one-quarter of healthy adults.10
Autoimmune diseases are relatively common, with an estimated prevalence of 20 million to 50 million in the United States, including 14% to 25% of patients with lung cancer (Table 2).11
Autoimmune disease incidence increases with age,12
so these conditions may disproportionately affect individuals with lung cancer.13
Table 2. Prevalence of Autoimmune Disease Among Individuals With Lung Cancer
| Clinical Characteristic | Percentage with Autoimmune Disease |
|---|---|
| Overall | 13.5 |
| Age | |
| < 75 years old | 12.3 |
| 75 to 84 years old | 14.7 |
| ≥ 85 years old | 13.8 |
| Gender | |
| Female | 16.8 |
| Male | 10.7 |
| Lung Cancer Stage | |
| Stage 1 |
17.5 |
| Stage 2 | 15.2 |
| Stage 3 | 13.0 |
| Stage 4 | 12.0 |
ICI clinical trials have excluded patients with a spectrum of autoimmune disease status, such as patients with “active” autoimmune disease (e.g., requiring prednisone equivalent >10 mg daily),14
patients with any history of autoimmune disease,15
or patients with “high-risk” autoimmune disease (allowing enrollment of individuals with “low-risk” autoimmune disease, such as psoriasis).16
Underlying these eligibility considerations are concerns that ICIs could induce an autoimmune disease flare, which could range from worsening rash and pruritus (psoriasis) to phrenic nerve dysfunction, diaphragmatic paralysis, and respiratory failure (myasthenia gravis).
Filling the void of prospective data, a number of observational studies have reported outcomes of ICI use in patients with autoimmune disease. A wide range of clinical patterns has been observed, including autoimmune disease flares in one-fourth of patients (generally minor and responsive to standard immunosuppression) without increased risk of irAE17
; increased incidence of low-grade irAE18
,19
,20
; and potential for rapid, fatal, clinical progression of some autoimmune conditions such as multiple sclerosis.21
On the basis of the available data, it appears that, for patients with autoimmune disease, ICI use is feasible with close clinical monitoring, particularly if the autoimmune disease is considered low-risk or inactive. However, for patients with more severe autoimmune disease, with the potential for flares that could adversely affect pulmonary, cardiac, or neurologic function, ICI administration should be considered high-risk and potentially fatal. ICI use is therefore recommended only after multidisciplinary discussion and planning among the entire clinical care team.
End-Organ Dysfunction
Depending on the specific agent, administration of conventional cytotoxic chemotherapy may require stringent organ-function thresholds. ICI use may be more feasible in some cases than others. In patients with mild renal, cardiac, and/or liver dysfunction, ICI administration does not appear to worsen baseline organ function and is well tolerated.22
Conversely, pulmonary function—which is rarely impacted by lung cancer chemotherapy regimens—may be affected by ICIs. Because more than 80% of patients with lung cancer are active or former smokers, comorbid lung disease is relatively common in this population. Numerous studies have demonstrated that interstitial lung disease increases risk of immune-mediated pneumonitis.23
,24
,25
The association between chronic obstructive pulmonary disease and pneumonitis risk is less clear.26
,27
Chronic Immunosuppression and Solid-Organ Transplant Recipients
Immunosuppression represents the mainstay of long-term management after solid-organ transplantation, preventing graft rejection and preserving graft function. This clinical scenario has implications for both the safety and the efficacy of ICIs. Retrospective studies have suggested that baseline use of corticosteroids may be associated with inferior outcomes from ICIs.28
,29
However, the poor prognosis associated with certain cancer-related steroid indications—such as symptomatic brain metastases, spinal cord compression, anorexia, and intractable pain—may underlie much of this effect.30
As for the safety of ICIs in this population, the largest study reports transplant rejection in almost 40% of cases as the most common cause of death.31
Single-case reports have described instances of ICI antitumor efficacy in transplant recipients, without compromising graft function.31
Availability of alternate sources of organ function seems highly relevant to this high-risk clinical scenario. That is, the threshold for considering ICIs in patients with kidney transplants (who have the option of hemodialysis in the setting of organ rejection) may differ from heart or liver transplant recipients.
Conclusions
With few ongoing or completed clinical trials evaluating the safety and efficacy of ICIs in patients with comorbidities, clinicians are left depending on retrospective studies of ICI use in patients with autoimmune disease, organ dysfunction, or organ transplants to guide their medical decision-making. However, with the decision to observe and report cases coming only after clinical outcomes are apparent, such studies are highly susceptible to publication bias. On the basis of the limited evidence currently available, we propose a clinical approach to ICI therapy in these populations (Fig.). ICI use in patient groups not addressed in this commentary—including elderly individuals; those with HIV, hepatitis C virus, or hepatitis B virus; and those with poor performance status—is summarized in other recent reviews.32
Fig. Suggested Algorithm for Treating Lung Cancer With Immune Checkpoint Inhibitors (ICIs) in Patients With Comorbidities
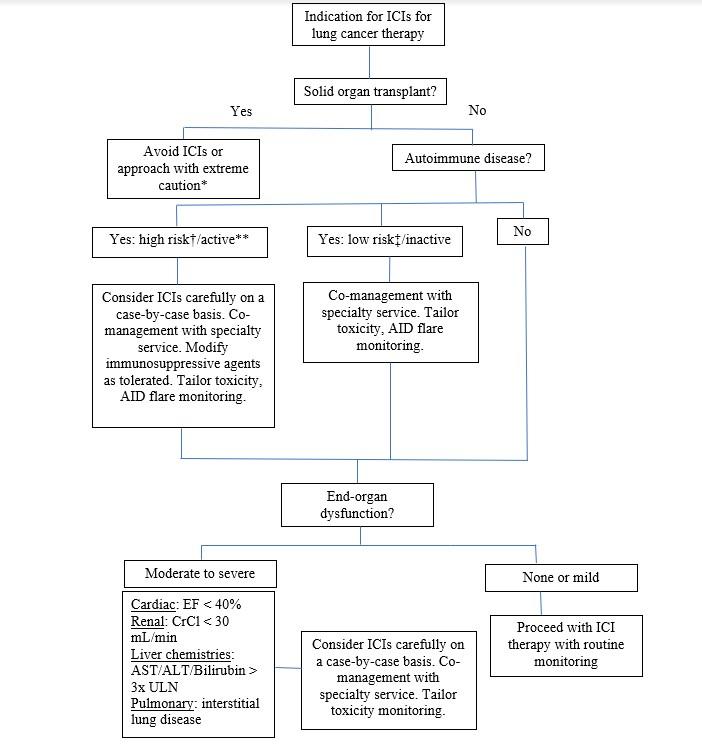
*In rare cases, potential exception for kidney transplant. **Receiving prednisone ≥ 10 mg daily or other immunosuppressive agents. †For example: myasthenia gravis, multiple sclerosis. ‡For example: psoriasis, rheumatoid arthritis.
- 1. Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. 2020;3(3):e200423.
- 2. Eggermont AMM, Crittenden M, Wargo J. Combination immunotherapy development in melanoma. Am Soc Clin Oncol Educ Book. 2018;38:197-207.
- 3. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168.
- 4. von Itzstein MS, Khan S, Gerber DE. Investigational biomarkers for checkpoint inhibitor immune-related adverse event prediction and diagnosis. Clin Chem. 2020;66(6):779-793.
- 5. Khan S, von Itzstein MS, Lu R, et al. Late-onset immunotherapy toxicity and delayed autoantibody changes: checkpoint inhibitor-induced Raynaud’s-like phenomenon. Oncologist. 2020;25(5):e753-e757.
- 6. Yoo SH, Keam B, Kim M, Kim TM, Kim DW, Heo DS. Generalization and representativeness of phase III immune checkpoint blockade trials in non-small cell lung cancer. Thoracic Cancer. 2018;9(6):736-744.
- 7. Schulkes KJ, Nguyen C, van den Bos F, van Elden LJ, Hamaker ME. Selection of patients in ongoing clinical trials on lung cancer. Lung. 2016;194(6):967-974.
- 8. Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930-1939.
- 9. Hsiehchen D, Watters MK, Lu R, Xie Y, Gerber DE. Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw Open. 2019;2(9):e1911519.
- 10. Wandstrat AE, Carr-Johnson F, Branch V, et al. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun. 2006;27(3):153-160.
- 11. a. b. Khan SA, Pruitt SL, Xuan L, Gerber DE. Prevalence of autoimmune disease among patients with lung cancer: implications for immunotherapy treatmentoptions. JAMA Oncol. 2016;2(11):1507-1508.
- 12. Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69(10):1615-1623.
- 13. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer stat facts: lung and bronchus cancer. Accessed August 13, 2020. https://seer.cancer.gov/statfacts/html/lungb.html
- 14. Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol. 2017;35(34):3851-3858.
- 15. Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924-3933.
- 16. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322.
- 17. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36(19):1905-1912.
- 18. Cortellini A, Buti S, Santini D, et al. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. Oncologist. 2019;24(6):e327-e337.
- 19. Kartolo A, Sattar J, Sahai V, Baetz T, Lakoff JM. Predictors of immunotherapy-induced immune-related adverse events. Current Oncol. 2018;25(5):e403-e410.
- 20. Danlos FX, Voisin AL, Dyevre V, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer. 2018;91:21-29.
- 21. Garcia CR, Jayswal R, Adams V, Anthony LB, Villano JL. Multiple sclerosis outcomes after cancer immunotherapy. Clin Translational Oncol. 2019;21(10):1336-1342.
- 22. Kanz BA, Pollack MH, Johnpulle R, et al. Safety and efficacy of anti-PD-1 in patients with baseline cardiac, renal, or hepatic dysfunction. J Immunother Cancer. 2016;4:60.
- 23. Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung Cancer. 2018;125:212-217.
- 24. Shimoji K, Masuda T, Nakanishi Y, et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-NSCLC cancers. J Clin Oncol. 2020;38(15)(suppl).
- 25. Nakanishi Y, Masuda T, Yamaguchi K, et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir Investig. 2019;57(5):451-459.
- 26. Okada N, Matsuoka R, Sakurada T, et al. Risk factors of immune checkpoint inhibitor-related interstitial lung disease in patients with lung cancer: a single-institution retrospective study. Sci Rep. 2020;10(1):13773.
- 27. Suzuki Y, Inui N, Karayama M, et al. Effect of PD-1 inhibitor on exhaled nitric oxide and pulmonary function in non-small cell lung cancer patients with and without COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1867-1877.
- 28. Taniguchi Y, Tamiya A, Isa SI, et al. Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Research. 2017;37(10):5857-5862.
- 29. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872-2878.
- 30. Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. 2019;37(22):1927-1934.
- 31. a. b. Fisher J, Zeitouni N, Fan W, Samie FH. Immune checkpoint inhibitor therapy in solid organ transplant recipients: a patient-centered systematic review. J Am Acad Dermatol. 2020;82(6):1490-1500.
- 32. von Itzstein MS, Gonugunta AS, Mayo HG, Minna JD, Gerber DE. Immunotherapy use in patients with lung cancer and comorbidities. Cancer J. 2020;26(6):525-536.






