Approximately one-third of patients with NSCLC are diagnosed with unresectable, locally advanced (LA) stage III disease.1 Historically, the standard of care for these patients has been concurrent platinum-based chemotherapy with definitive thoracic radiation therapy (cCRT), 2 garnering a median OS of approximately 28 months and 5-year OS rates of 15% to 30%, at best.
The advent of immunotherapy has resulted in a revolution in the treatment paradigm for patients with LA-NSCLC, with a significant and meaningful improvement in outcomes achieved through the incorporation of the PD-L1 inhibitor durvalumab as a consolidation strategy after cCRT.3 ,4 Updated results from the PACIFIC trial5 have confirmed the sustained benefit of durvalumab following cCRT, leading to a 28% reduction in the risk of death (OS HR 0.72, 95% CI [0.59-0.89]), with an estimated 42.9% of patients surviving 5 years (vs. 33.4% for cCRT alone), and an unprecedented median OS of 47.5 months (vs. 29.1 months for cCRT alone). Overall, the results of the PACIFIC trial represent a major breakthrough in the treatment of LA-NSCLC, and durvalumab has now been widely adopted as the standard-of-care therapy for patients with LA-NSCLC that has not progressed after concurrent chemoradiation.6
Given the results of the PACIFIC trial, the next logical step has been to investigate whether the use of immune checkpoint inhibitors (ICIs) earlier in the course of the disease, alongside cCRT, could increase the depth and duration of response, leading to prolonged clinical benefit for a broader number of patients.
There is considerable evidence that supports this strategy: first, the heightened response rates observed when pembrolizumab is given with chemotherapy in the advanced disease setting compared to chemotherapy alone7; second, the preclinical and clinical evidence suggesting that the effectiveness of pembrolizumab is enhanced when the drug is combined with radiotherapy8; and finally, the demonstrated potential of radiation and chemotherapy to up-regulate PD-L1 expression on tumor cells, thereby priming tumors for sensitivity to ICIs.9 This strategy might be further justified by the results of an exploratory, post-hoc subgroup analysis from PACIFIC that suggests the benefit of earlier initiation of consolidative durvalumab within 14 days of cCRT.10 Moreover, the earlier initiation of ICIs with cCRT could potentially extend the clinical benefit to those patients who would not otherwise satisfy criteria for durvalumab consolidation because of disease progression either during or immediately after cCRT.11
One of the major concerns regarding the use of ICIs combined with thoracic radiotherapy relates to safety, specifically the potential risk of increased pneumonitis, as both therapies are associated with pulmonary toxicity. Early phase I trials suggested a slightly increased risk of grade 3–4 pneumonitis with the addition of ICIs during cCRT. In a small (n = 21) phase I trial of pembrolizumab given concurrently with definitive cCRT,12 there was a 9.5% incidence of grade 3–5 pneumonitis when the volume of lung parenchyma that received >20 Gy of radiation (V20) was restricted to 31% or less. Similarly, in the NICOLAS-ETOP trial, grade 3 pneumonitis events were reported in 10% of patients treated with reduced doses of nivolumab concurrent with cCRT.13 To put these results into perspective, in the PACIFIC trial, grade 3–4 pneumonitis or radiation pneumonitis occurred in 3.4% and 2.6% of patients, respectively. It is important to bear in mind that, in the PACIFIC trial, immunotherapy was given sequentially, patients with pre-existing pneumonitis of at least grade 2 after cCRT were ineligible for random assignment to , and the recommended V20 constraint was less stringent, at 35% or lower.3
KEYNOTE-799 is a phase II, non-randomized trial designed to investigate the efficacy and safety of pembrolizumab given concurrently with cCRT in a larger cohort of patients with unresectable stage III LA-NSCLC.14
The study, which launched in November 2018 and completed enrollment in July 2020, included patients 18 years or older diagnosed with treatment-naive, unresectable, pathologically and/or radiologically confirmed stage IIIA/IIIB/IIIC NSCLC. Participants were required to have an ECOG performance status of 0-1 and adequate pulmonary function. Tumor radiation treatment plans likely to encompass a V20 of 31% or less were stipulated.
Tissue samples were required to determine PD-L1 tumor proportion score (TPS), although patients were enrolled regardless of PD-L1 status. In the design of the trial, it was hypothesized that the response to cCRT and pembrolizumab would be agnostic of PD-L1 status because of the effects of radiotherapy in overcoming an immunosuppressive tumor microenvironment, leading to PD-L1 upregulation.9
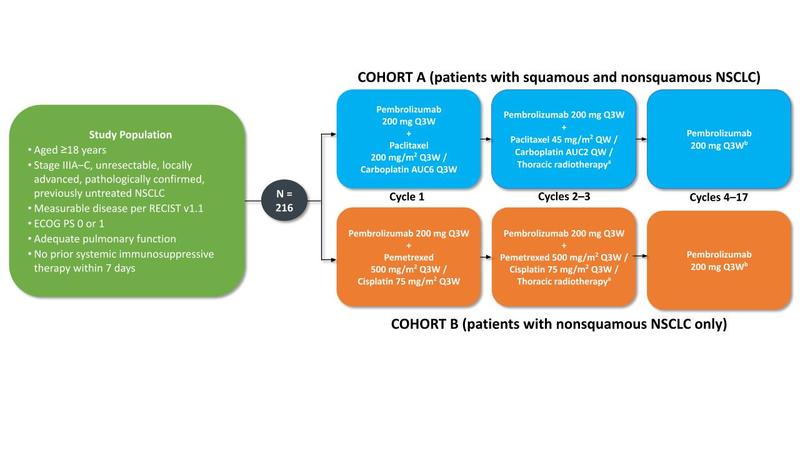
The study had two arms. Patients in cohort A (who had either squamous or nonsquamous disease) received one 3-week cycle of carboplatin (AUC 6), paclitaxel (200 mg/m2), and pembrolizumab (200 mg), followed by carboplatin (AUC 2) and paclitaxel (45 mg/m2) once weekly for 6 weeks concurrent with two 3-week cycles of pembrolizumab (200 mg) and standard thoracic radiotherapy. Patients in cohort B (who exclusively had nonsquamous disease) received cisplatin (75 mg/m2), pemetrexed (500 mg/m2), and pembrolizumab (200 mg) every 3 weeks for three cycles, with thoracic radiotherapy given during cycles 2 and 3. After cCRT, patients in both cohorts received 14 additional 3-week cycles of pembrolizumab 200 mg for a total of 17 cycles (1 year). Thoracic radiotherapy was administered 5 days per week in once-daily fractions of 2 Gy to a target dose of 60 Gy in 30 fractions using standardized radiotherapy techniques. The study design is summarized in the Fig.
Fig. Study Design of the KEYNOTE-799 Trial.
Primary objectives: ORR per RECIST version 1.1 by blinded, independent central review; percentage of patients who develop grade ≥3 pneumonitis. Secondary objectives: PFS, OS, safety. Figure courtesy of Noemi Reguart.
Abbreviations: PE, primary efficacy; PS, performance status; QW, every week; Q3W, every 3 weeks.
a60 Gy in 30 daily 2-Gy fractions. bTreatment will continue until cycle 17 is completed or until documented disease progression, unacceptable adverse events, intercurrent illness that prevents further administration of treatment, or study withdrawal. Pembrolizumab therapy will be discontinued permanently in patients who develop grade ≥3 or recurrent grade 2 pneumonitis.
The coprimary end points of the study were objective response rate (ORR) per RECIST v1.1 by blinded independent central review (BICR) and the incidence of grade 3 to 5 pneumonitis. Secondary objectives were PFS per RECIST v1.1 by BICR, OS, and safety.
Preliminary results of KEYNOTE-799 were first presented at the 2020 Annual Meeting of the American Society of Clinical Oncology,15 when the study was still recruiting patients, with a short follow-up of 8.3 months (cohort A, n = 112) and 5.8 months (cohort B, n = 73). Across both cohorts, most patients were former or current smokers and had stage IIIB disease. Among tumor samples evaluable for PD-L1 expression, PD-L1 TPS was ≥1% in 58.9% and 39.2% in cohorts A and B, respectively. Initial antitumor activity was promising, with an ORR of 67.7% (90% CI [58.9−74.3]) in cohort A and 56.6% in cohort B (90% CI [44.4−68.2]). For most patients, the responses were durable at the time of analysis.15
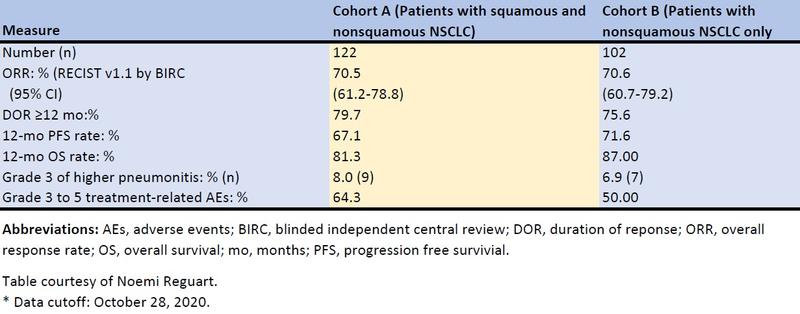
In the last cutoff report,16 with the enrollment in both cohorts already completed and a longer follow-up (cohort A, n = 112, 18.5 months; cohort B, n = 102, 13.7 months), pembrolizumab plus cCRT continued to demonstrate robust antitumor activity with responses exceeding 70% in both groups regardless of PD-L1 status (ORR by TPS <1% and TPS ≥1%, respectively: cohort A, 66.7% and 75.8%; cohort B, 71.4% and 72.5%) or tumor histology (ORR squamous, 71.2% and nonsquamous, 69.2%). The median duration of response (DOR), PFS, and OS were immature because many patients were still receiving study therapy, but it was estimated that >75% were still responding at 1 year, with a 1-year PFS of approximately 70% and 1-year OS rates higher than 80% in both cohorts. The results are summarized in the Table.
Table. Summary of Efficacy in the KEYNOTE-799 Trial.
The incidence of adverse events (AEs) was consistent with the established toxicity profiles of cCRT for stage III NSCLC and for pembrolizumab monotherapy, with grade 3–5 treatment-related AEs occurring in 64.3% and 50.0% of patients in cohorts A and B, respectively. The incidence of grade 3 or higher pneumonitis was in the expected range for ICIs and cCRT combinations, reaching 8.0% (9 of 112 patients) in cohort A and 6.9% (7 of 102 patients) in cohort B, with a median time to onset of first pneumonitis of approximately 4 months in both cohorts. There were four (3.6%) fatal treatment-related AEs in cohort A, all due to grade 5 pneumonitis, and one (1%) in cohort B due to grade 5 interstitial lung disease. Pneumonitis was the most frequent treatment-related AE leading to discontinuation of treatment in either cohort.16
The KEYNOTE-799 trial successfully demonstrated robust efficacy and a manageable safety profile of pembrolizumab combined with cCRT in stage III LA-NSCLC; it suggests, but does not yet prove, that there is an additional benefit of administering anti-PD-(L)1 therapies alongside cCRT. Seeking to build upon that evidence is the KEYLYNK-012 study (NCT04380636), an ongoing phase III trial evaluating the efficacy of pembrolizumab in combination with cCRT followed by pembrolizumab with or without the PARP inhibitor olaparib compared with the current standard-of-care PACIFIC regimen (cCRT followed by durvalumab). The co-primary endpoints include PFS (assessed via BIRC) and OS.
Several other ongoing phase III studies are investigating the use of ICIs combined with cCRT in stage III LA-NSCLC: CheckMate73L (NCT04026412) is a three-arm study of nivolumab plus cCRT followed by consolidation consisting of nivolumab with or without ipilimumab versus consolidation with durvalumab; the ECOG-ACRIN EA5181 study (NCT04092283) randomly assigns patients to cCRT with or without durvalumab followed by consolidation durvalumab; and the PACIFIC-2 trial (NCT03519971) is comparing durvalumab concomitantly with CRT followed by durvalumab consolidation until disease progression or discontinuation versus a control arm receiving cCRT alone without consolidation immunotherapy.
Similarly, other studies are working to evaluate novel immunotherapies given concurrently with cCRT. The PACIFIC-8 trial will evaluate domvanalimab, a novel anti-TIGIT antibody, with durvalumab plus cCRT in patients with LA-NSCLC. Another ongoing phase III trial (NCT04866017) will evaluate the activity of tislelizumab (a PD-1 antibody) plus BGB-A1217 (an anti-TIGIT antibody) co-administered with cCRT in the same setting of LA-NSCLC.
Treatment for LA-NSCLC is evolving rapidly; as with anything new, these novel protocols invite questions and challenges: Does it make sense to consider immunotherapy prior to initiating concurrent chemoradiation? Which is the optimal duration of consolidation treatment? Can we improve outcomes by prolonging consolidation therapy beyond one year? other ICIs? What are the effects of different radiation therapy techniques and V20 constraints on immunotherapy-related toxicities? How can we ensure the accuracy of both response assessment and confirmation of disease progression? How do we conduct work-ups that differentiate among the broad diversity of diagnoses (infection, toxicity, fibrosis, tumor progression) in a patient presenting with respiratory symptoms during the course of treatment? Can we better predict outcomes and relapses by evaluating circulating tumor biomarkers?
Clearly, numerous questions remain, and answers are needed. However, given the advances observed to date since the routine institution of immunotherapy in LA-NSCLC, we anticipate further progress in the years ahead.
- 1. Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2017;12(7):1109-1121.
- 2. Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181-2190.
- 3. a. b. Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181-2190.
- 4. Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350.
- 5. Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. J Clin Oncol. 2021;39(15_suppl):8511.
- 6. Remon J, Soria JC, Peters S. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021 Aug 28. (Epub ahead of print).
- 7. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078-2092.
- 8. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895-903.
- 9. a. b. Gong X, Li X, Jiang T, et al. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12(7):1085-1097.
- 10. Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol. 2021;16(5):860-867.
- 11. Horinouchi H, Atagi S, Oizumi S, et al. Real-world outcomes of chemoradiotherapy for unresectable Stage III non-small cell lung cancer: The SOLUTION study. Cancer Med. 2020;9(18):6597-6608.
- 12. Jabbour SK, Berman AT, Decker RH, et al. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol. 2020;6(6):848-855.
- 13. Peters S, Felip E, Dafni U, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer. 2019;133:83-87.
- 14. Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol. 2021; 7(9):1351-1359
- 15. a. b. Jabbour SK, Lee KH, Frost N, et al. Phase II study of pembrolizumab (pembro) plus platinum doublet chemotherapy and radiotherapy as first-line therapy for unresectable, locally advanced stage III NSCLC: KEYNOTE-799. J Clin Oncol. 2020;38(15_suppl):9008.
- 16. a. b. Jabbour SK, Lee KH, Frost N, et al. KEYNOTE-799: Phase 2 trial of pembrolizumab plus platinum chemotherapy and radiotherapy for unresectable, locally advanced, stage 3 NSCLC. J Clin Oncol. 2021;39(15_suppl):8512.