By Vassiliki Papadimitrakopoulou, MD
Posted: June 2018
The Lung Master Protocol Trial (Lung-MAP) was designed in 2014 as a phase II/III trial overseen by a private–public collaboration among institutions participating in the National Cancer Institute’s (NCI’s) National Clinical Trials Network, the Foundation for the National Institutes of Health (FNIH), patient advocacy organizations, and numerous pharmaceutical companies. It uses an umbrella design that enables the conduct of multiple complex clinical trials targeting different biomarkers using one overarching protocol.1 The trial leverages molecular profiling of tumors to identify and treat patients with histologically common (squamous cell lung cancer) but molecularly diverse tumors within independent substudies designed to provide a registration potential. Thus, it overcomes the difficulties of accruing patients with rare subsets of molecular alterations onto individual trials.
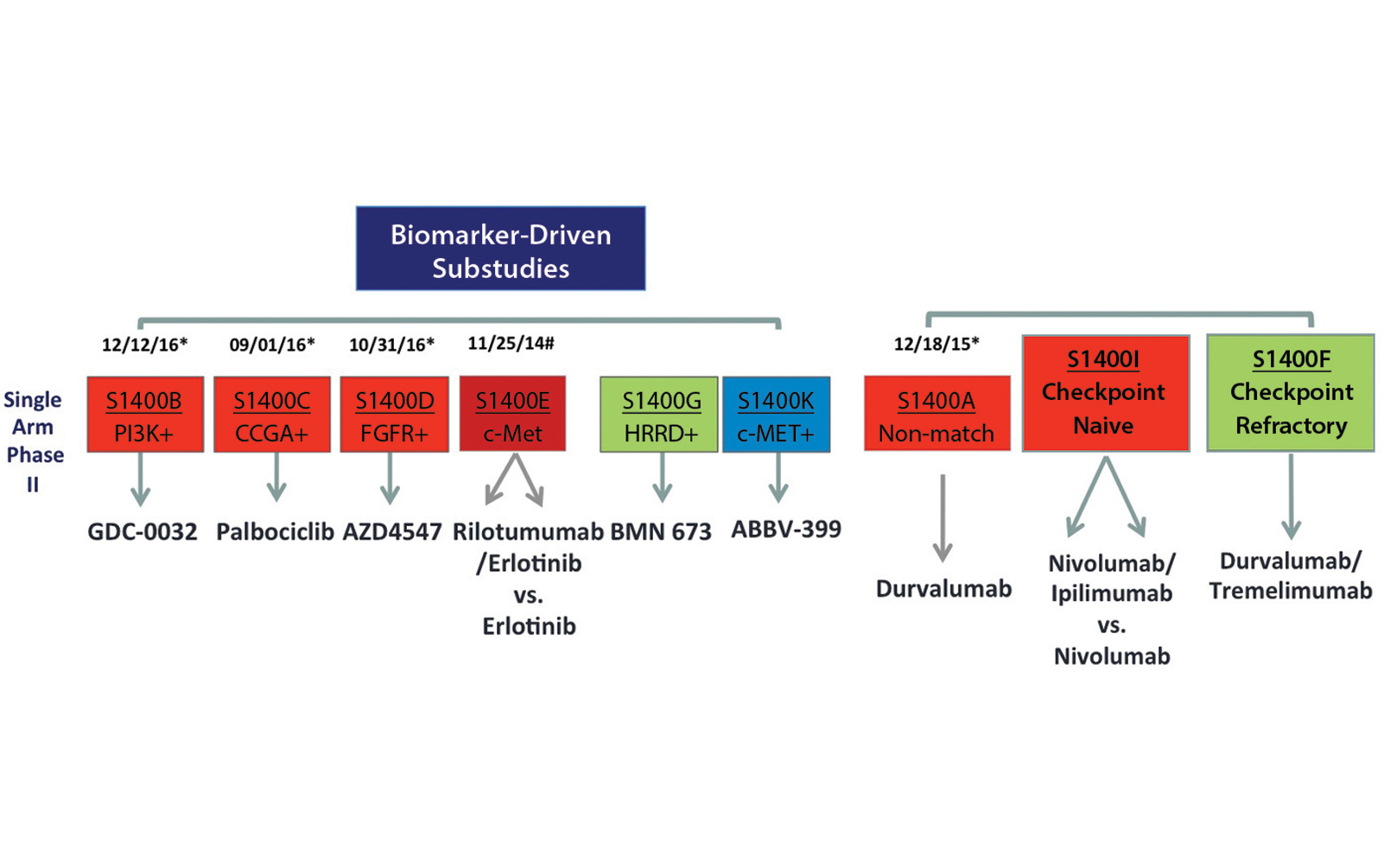
When the trial was activated in June 2014, Lung-MAP originally had four biomarker-driven substudies and one nonmatch substudy. The biomarker-driven substudies independently evaluated:
1. Taselisib for PIK3CA mutations [S1400B],
2. Palbociclib for CDK4/6 alterations [S1400C],
3. AZD4547 for FGFR alterations [S1400D], and
4. Rilotumumab and erlotinib for c-MET overexpression [S1400E].
Patients who did not have any of those alterations were enrolled in the non-match substudy evaluating durvalumab, an anti–PD-L1 immunotherapy agent. Initially, the first three substudies included docetaxel as a randomized standard-of-care control arm. The fourth study using erlotinib as the control arm was closed in September 2014. Since trial activation in June 2014, the protocol has been amended multiple times to stay current with advances in lung cancer treatment and to remove the standard-of-care control arm from the master protocol. The trial was also modified from a phase II/III trial to one that includes both phase II and phase III substudies. The phase II studies allow investigational agents to be evaluated in single-arm trials and improve the efficiency of assessment if these drugs are found to be either highly effective or ineffective.
Adaptation to Change
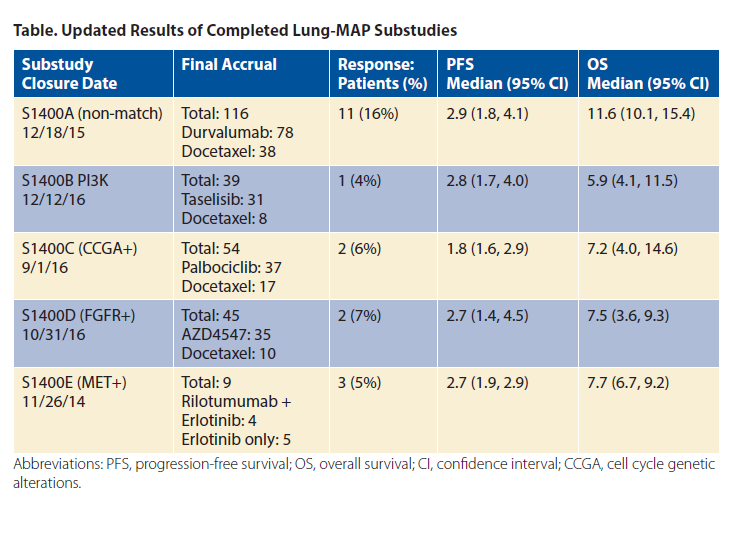
Lung-MAP is open at more than 700 sites in the United States and Canada. As of January 5, 2018, 1,407 patients have been registered to screening; 1,244 patients have biomarker results, and 529 patients have registered to a substudy. The results of completed substudies have been presented and are included in summary in the table.2-6 The prevalence of putative driver alterations is similar to the incidence previously described in primarily early stage, resected cohorts such as The Cancer Genome Atlas. The current schema of the protocol is shown in the figure.
Dr. Vassiliki Papadimitrakopoulou

Three new substudies (S1400G, S1400K, and S1400F) are now part of Lung-MAP. S1400G evaluates talazoparib (PARP inhibitor) for homologous recombination repair deficiency, S1400F evaluates combination durvalumab and tremelimumab (anti–CTLA-4) in patients with immune checkpoint– refractory disease without a biomarker match, and S1400K evaluates ABBV-399 (antibody–drug conjugate) for c-MET–overexpressing tumors. More recent modifications to the trial are intended to allow all histologies to be eligible and to add screening biomarkers for immunotherapy combinations as well as exploratory biomarker testing. These changes along with clinical trials specifically targeting immune checkpoint inhibitor resistance are expected in upcoming trial revisions.
Additionally, the rapidly changing treatment landscape of NSCLC demands flexibility and adaptability in a master protocol. The substudy modularity inherent in the Lung-MAP design has allowed the protocol to adapt efficiently to rapid changes in the standard of care with the relatively recent approvals of nivolumab and other PD-1/PD-L1 inhibitors for the treatment of lung squamous cell carcinoma in the second-line setting. As described above, the matched substudies were converted into single-arm phase II trials while retaining a pathway for regulatory drug approval. The flexibility of the substudy concept has also enabled the development of a new combination immunotherapy option for patients with check point-refractory disease without a biomarker match. As our understanding of intrinsic and acquired immune checkpoint inhibitor resistance evolves and more precise determinants of response beyond tumor PD-L1 expression are identified, it is envisioned that more personalized, biomarker-driven immunotherapy trials will become part of the Lung-MAP design. ✦
About the Author: Dr. Papadimitrakopoulou is a professor of medicine in the Department of Thoracic/Head and Neck Medical Oncology at The University of Texas MD Anderson Cancer Center.
References:
1. Herbst RS, Gandara DR, Hirsch FR, et al. Lung Master Protocol (Lung-MAP)-A Biomarker- Driven Protocol for Accelerating Development of Therapies for Squamous Cell Lung Cancer: SWOG S1400. Clin Cancer Res. 2015;21:1514- 1524.
2. Herbst R, Redman M, Gandara DR, et al. OA 14.07 Progress in Lung Squamous Cell Carcinoma from the Lung-MAP Master Protocol (S1400) Sub-Studies S1400A, S1400B, S1400C and S1400D. J Thorac Oncol. 12:S1783-S1784.
3. Wade JL, Langer CJ, Redman M, et al. A phase II study of GDC-0032 (taselisib) for previously treated PI3K positive patients with stage IV squamous cell lung cancer (SqNSCLC): LUNGMAP sub-study SWOG S1400B. J Clin Oncol. 2017;35:9054-9054.
4. Edelman MJ, Redman MW, Albain KS, et al. A phase II study of palbociclib (P) for previously treated cell cycle gene alteration positive patients (pts) with stage IV squamous cell lung cancer (SCC): Lung-MAP sub-study SWOG S1400C. J Clin Oncol. 2017;35:9056-9056.
5. Aggarwal C, Redman MW, Lara P, et al. Phase II study of the FGFR inhibitor AZD4547 in previously treated patients with FGF pathway activated stage IV squamous cell lung cancer (SqNSCLC): LUNG-MAP sub-study SWOG S1400D. J Clin Oncol. 2017;35:9055-9055.
6. Papadimitrakopoulou V, Redman MW, Borghaei H, et al. A phase II study of durvalumab (MEDI4736) for previously treated patients with stage IV squamous NSCLC (SqNSCLC): Lung- MAP Sub-study SWOG S1400A. Ann Oncol. 2017;28.