Most cancer immunotherapies currently in development aim to stimulate antitumor T-cell responses either by adoptive transfer of in vitro expanded native or genetically modified T lymphocytes targeting malignant cells,1
,2
administration of neutralizing monoclonal antibodies (mAb) specific to T-cell inhibitory receptors, such as cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed death-1 (PD-1),3
,4
,5
,6
,7
or therapeutic vaccination of cancer patients with tumor-associated antigens (TAA).8
,9
,10
In this regard, identification of cancer-specific peptide antigens, by analysis of tumor-reactive cytotoxic T lymphocytes (CTL) derived from cancer patients, has been used to design various therapeutic vaccines, including for the treatment of lung cancer.8
Lung Cancer Vaccines: History and New Trends
Optimal cancer vaccines include those that rely on DNA sequences or RNA molecules, as well as synthetic long peptides derived from TAA, such as cancer testis antigens, viral antigens, and mutant antigens, delivered with an adjuvant or pulsed on autologous or allogeneic dendritic cells (DC).11
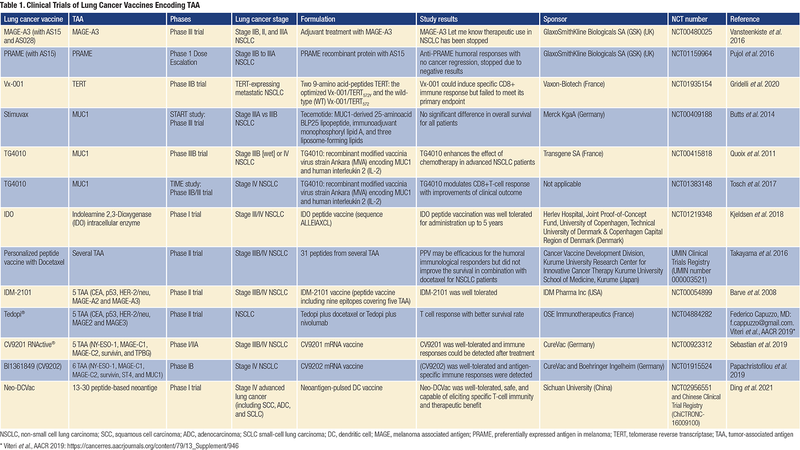
Initial TAA-based therapeutic vaccines showed low toxicity profiles and good tolerability. One vaccine developed to treat lung cancer by GlaxoSmithKline (GSK) used the MAGE-A3 antigen. In a phase II trial in patients with lung cancer who had undergone surgery to remove their tumors, this vaccination reduced the risk of relapse after 28 months by 27%. The reduction was not statistically significant, as there were only 182 patients in the trial, but it trended in the right direction. GSK then conducted a phase III trial in over 2,000 patients with early-stage lung cancer. Unfortunately, adjuvant treatment with the MAGE-A3 immunotherapeutic did not increase disease-free survival (DFS) compared with placebo in patients with MAGE-A3-positive surgically resected NSCLC.12
Another therapeutic vaccination conducted with the PRAME antigen demonstrated anti-PRAME humoral responses, but because a similar treatment had not revealed objective cancer regression nor an increase in DFS in patients with NSCLC, further development was stopped.13
More recently, a vaccine that targets the telomerase reverse transcriptase was tested in a phase IIB trial in patients with NSCLC who are positive for the HLA-A2 antigen. Although the vaccine could induce specific CD8 T-cell responses, it failed to improve patient OS.14
Furthermore, a peptide vaccine from Merck KgaA (Stimuvax) sought to stimulate the immune system against the mucin (MUC)1 antigen. In a stage III trial including 1,300 patients with inoperable NSCLC, the primary endpoint of OS did not differ between placebo and vaccine with cyclophosphamide. However, in a subgroup analysis, patients who received vaccine after concurrent chemoradiotherapy had a 10.2-month improvement in median survival.15
In addition, a phase IIB trial with TG4010 vaccine (viral vaccine comprising human MUC1 and interleukin [IL]-2) and first-line chemotherapy was conducted by Transgene in 148 patients with advanced NSCLC. Six-month PFS was 43% in the TG4010-plus-chemotherapy group and 35% in the chemotherapy-alone group, suggesting that TG4010 could enhance the effects of chemotherapy.16
A phase IIB-III trial was then conducted, and data supported the clinical value of TG4010 plus chemotherapy.17
In another study, TG4010 improved survival in some patients with NSCLC and correlated with induction of CTL responses against targeted and non-targeted TAA.18
Moreover, an indoleamine 2,3-dioxygenase (IDO) peptide vaccine showed durable clinical responses on long-term follow-up in patients with stage III-IV NSCLC.19
In contrast, a personalized multi-peptide vaccination (up to 31 peptides from several TAAs) for patients with previously treated advanced NSCLC did not improve survival in combination with docetaxel, although a humoral immunological response was observed.20
Among multi-epitopic therapeutic vaccines in NSCLC, the IDM-2101 vaccine (peptide vaccine including nine epitopes covering five TAAs overexpressed in lung cancer and the universal helper pan-DR epitope), administered as monotherapy to patients with pre-treated HLA-A2, yielded some efficacy in terms of survival.21
Another active immunotherapy approach has been tested by OSE Immunotherapeutics using 10 epitopes selected from five TAAs. A phase 2 clinical trial conducted in patients who had NSCLC with a poor prognosis showed a better survival rate, which correlated with T-cell epitope responses. A phase 3 trial in patients with NSCLC who are positive for the HLA-A2 antigen, after the failure of PD-1 blockade, was then conducted and has shown some benefit in OS (Table 1).22
A mRNA-based vaccine encoding five NSCLC antigens conducted in a phase I/IIa trial demonstrated immune responses supporting further clinical investigation.23
Another mRNA vaccine encoding six NSCLC TAAs co-delivered with protamine/mRNA complexes that had self-adjuvant properties was also tested and some responses were observed.24
Perspectives and Future Direction
Although new treatment avenues are opening up for NSCLC, including TAA-based cancer vaccines, most phase III clinical trials have not yet demonstrated survival benefits, despite the promising results achieved in preliminary phase II randomized trials. Indeed, the therapeutic benefit of currently available therapies remains limited in NSCLC, which still represents the major cause of cancer-related mortality worldwide, and cancer vaccines have not been successful in particular in late-stage patients with treatment-refractory tumors.9
,25
A recent pilot study conducted in 12 patients with metastatic lung cancer who received a personalized vaccine based on neoantigen peptide-pulsed autologous DC also demonstrated limited objective effectiveness.26
As a general rule, only a fraction of patients with cancer have responded to current therapeutic interventions, most likely due to multiple pre-existing and acquired mechanisms leading to tumor escape from T-cell immunity.27
,28
Among resistance mechanisms, expression of inhibitory receptors on vaccine-induced, tumor-specific CD8 T lymphocytes probably plays an important role.29
,30
Therefore, therapeutic cancer vaccines based on mutant neoantigens have been combined with immune checkpoint blockade (ICB) to promote proliferation of tumor-specific T lymphocytes and overcome T-cell exhaustion. Studies based on immunization of melanoma patients with personalized neoepitope peptide vaccines or RNA-based poly-neoepitope vaccines in combination with anti-PD-1 demonstrated tumor regression with expansion of the repertoire of neoantigen-specific T cells.31
,32
More recently, TAA RNA vaccines were combined with PD-1 blockade, and clinical responses, accompanied by the induction of strong CD4 and CD8 T-cell immunity against the vaccine’s non-mutated TAAs, were observed.33
Nevertheless, relapse associated with outgrowth of β2m-deficient tumor cells as an acquired mechanism of resistance was reported in responder patients.33
Indeed, downregulation of antigen presentation machinery (APM) components, such as the loss of β2m, is frequently used by tumors to evade CTL reactivity.
Among additional mechanisms involved in tumor resistance to CD8 T-cell immunity, alterations in transporter associated with antigen processing (TAP) play a major role by inducing a sharp decrease in surface expression of major histocompatibility complex-class I (MHC-I)/β2m-peptide complexes, enabling malignant cells to evade CTL-mediated killing.34
,35
,36
,37
,38
,39
Consistently, earlier studies from several groups, including ours, have focused on defects in antigen presentation machinery (APM) in cancer cells, in particular loss of MHC-I molecules and downregulation of TAP1 and/or TAP2 subunit, resulting in resistance to T-cell receptor (TCR)-dependent cytotoxicity.40
,41
Therefore, developing novel immunotherapies with tumor neoantigens that are selectively presented by cancer cells carrying defects in antigen processing, referred to as T-cell epitopes associated with impaired peptide processing (TEIPP), is a novel therapeutic option for patients with acquired resistance to cancer immunotherapies. TEIPP-specific T cells represent valuable resources to target immune-edited MHC-I-low tumors that have acquired resistance to CD8 T-cell immunity and presumably also to ICB.42
In this context, we previously identified the first human non-mutated tumor neoepitope recognized on MHC-I-low NSCLC tumor cells by an autologous CTL clone isolated from tumor-infiltrating lymphocytes of a lung cancer patient. This epitope (ppCT16-25) is derived from the preprocalcitonin (ppCT) signal sequence and is processed independently of TAP.40
,43
Additional human non-mutant neoantigens presented by TAP-deficient tumors were more recently identified using a combinatorial screening approach.44
These non-mutant neoepitopes, together with the ppCT16-25 neoepitope, represent attractive candidates for therapeutic cancer vaccines targeting immune-escaped tumor variants. Consistently, our group had provided in vitro and in vivo proofs of concept of an active cancer immunotherapy based on a cocktail of ppCT peptides, including the ppCT16-25 TEIPP, capable of inducing antitumor CTL responses in HLA-A*0201/HLA-DR3-transgenic mice and in NOD-scid-Il2rγnull mice adoptively transferred with human peripheral blood mononuclear cells, resulting in regression of established tumors expressing low levels of HLA-A2/human ppCT complexes.45
These results support the conclusion that TEIPP correspond to valuable shared neoepitopes to target cancer cells expressing low levels of HLA-class I molecules as a consequence of alteration in TAP expression upon antitumor CTL pressure.
Conclusions
The specificity of therapeutic vaccination combined with ICB offers an attractive avenue for the development of future cancer immunotherapies for patients with acquired resistance to current treatments. Non-mutant neoantigens, combined with shared TAAs and mutant neoepitopes, are well suited to target tumor heterogeneity in order to eliminate all types of malignant cells deficient or proficient for TAP expression. Combination of a TEIPP-based cancer vaccine with checkpoint inhibitors, such as anti-PD-(L)1 in NSCLC, can expand antigen-specific T lymphocytes and induce a broader T-cell specificity repertoire, overcoming T-cell exhaustion and resulting in elimination of malignant cells, including multi-resistant, immune-escaped tumor variants.
Disclosure: Dr. Mami-Chouaib is the founder of ElyssaMed, a developer of immunotherapy cancer vaccines.
- 1. Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257(1):56-71.
- 2. Barrett DM, Grupp SA, June CH. Chimeric Antigen Receptor- and TCR-Modified T Cells Enter Main Street and Wall Street. J Immunol. 2015;195(3):755-761.
- 3. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480-489.
- 4. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
- 5. Brahmer J, Reckamp KL, Baas P, et al.. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135.
- 6. Borghaei H., Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627-1639.
- 7. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550.
- 8. a. b. Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135-146.y
- 9. a. b. Melero I, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11(9):509-524.
- 10. Leko V, Rosenberg SA. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell. 2020;38(4):454-472.
- 11. Melief CJM, van Hall T, Arens R, et al. Therapeutic cancer vaccines. J Clin Invest. 2015;125(9):3401-3412.
- 12. Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(6):822-835.
- 13. Pujol JL, De Pas T, Rittmeyer A, et al. Safety and Immunogenicity of the PRAME Cancer Immunotherapeutic in Patients with Resected Non-Small Cell Lung Cancer: A Phase I Dose Escalation Study. J Thorac Oncol. 2016;11(12):2208-2217.
- 14. Gridelli C, Ciuleanu T, Domine M, et al. Clinical activity of a htert (vx-001) cancer vaccine as post-chemotherapy maintenance immunotherapy in patients with stage IV non-small cell lung cancer: final results of a randomised phase 2 clinical trial. Br J Cancer. 2020;122(10):1461-1466.
- 15. Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(1):59-68.
- 16. Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(1):59-68.
- 17. Quoix E, Lena H, Losonczy G, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17(2):212-223.
- 18. Tosch C, Bastien B, Barraud L, et al. Viral based vaccine TG4010 induces broadening of specific immune response and improves outcome in advanced NSCLC. J Immunother Cancer. 2017;5(1):70.
- 19. Kjeldsen JW, Iversen TZ, Engell-Noerregaard L, Mellemgaard A, Andersen MH, Svane IM.. Durable Clinical Responses and Long-Term Follow-Up of Stage III-IV Non-Small-Cell Lung Cancer (NSCLC) Patients Treated With IDO Peptide Vaccine in a Phase I Study-A Brief Research Report. Front Immunol. 2018;9:2145.
- 20. Takayama K, Sugawara S, Saijo Y, et al. Randomized Phase II Study of Docetaxel plus Personalized Peptide Vaccination versus Docetaxel plus Placebo for Patients with Previously Treated Advanced Wild Type EGFR Non-Small-Cell Lung Cancer. J Immunol Res. 2016;2016:1745108.
- 21. Barve M, Bender J, Senzer N, et al. Induction of immune responses and clinical efficacy in a phase II trial of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine, in metastatic non-small-cell lung cancer. J Clin Oncol. 2008;26(27):4418-4425.
- 22. Barve M, Bender J, Senzer N, et al. Induction of immune responses and clinical efficacy in a phase II trial of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine, in metastatic non-small-cell lung cancer. J Clin Oncol. 2008;26(27):4418-4425.
- 23. Sebastian M, Shroder A, Scheel B, et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol Immunother; 2019;68(5):799-812.
- 24. Papachristofilou A, Hipp MM, Klinkhardt U, et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J Immunother Cancer. 2019;7(1):38.
- 25. Romero P, Banchereau J, Bhardwaj N, et al. The Human Vaccines Project: A roadmap for cancer vaccine development. Sci Transl Med. 2016;8(334):334ps9.
- 26. Ding Z, Li Q, Zhang R, et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct Target Ther. 2021;6(1):26.
- 27. Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017;2:188-201.
- 28. Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375(9):819-829.
- 29. Leclerc M, Voilin E, Gros G, et al. Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat Commun. 2019;10(1):3345.
- 30. Baitsch L, Baumgaertner P, Devevre E, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350-2360.
- 31. Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217-221.
- 32. Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222-226.
- 33. a. b. Sahin U, Oehm P, Derhovanessian E, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107-112.
- 34. Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181-273.
- 35. Abele R, Tampe R. Modulation of the antigen transport machinery TAP by friends and enemies. FEBS Lett. 2006;580(4):1156-1163.
- 36. Setiadi AF, David MD, Seipp RP, Hartikainen JA, Gopaul R, Jefferies WA. Epigenetic control of the immune escape mechanisms in malignant carcinomas. Mol Cell Biol. 2007;27(22):7886-7894.
- 37. Einstein MH, Leanza S, Chiu LG, et al. Genetic variants in TAP are associated with high-grade cervical neoplasia. Clin Cancer Res. 2009;15(3):1019-1023.
- 38. Leibowitz M.S., Andrade Filho PA, Ferrone S, Ferris RL, et al. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother. 2011;60(4):525-535.
- 39. Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44-51.
- 40. a. b. Durgeau A, et al. Different expression levels of the TAP peptide transporter lead to recognition of different antigenic peptides by tumor-specific CTL. J Immunol. 2011;187(11):5532-5539.
- 41. Leone P, Shin E-C, Perosa F, Vacca A, Dammacco F, Racanelli V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst. 2013;105(16):1172-1187.
- 42. Marijt KA, Doorduijn EM, van Hall TT. TEIPP antigens for T-cell based immunotherapy of immune-edited HLA class Ilow cancers. Mol Immunol. 2019;113:43-49.
- 43. El Hage F., Stroobant V, Vergnon I, et al. Preprocalcitonin signal peptide generates a cytotoxic T lymphocyte-defined tumor epitope processed by a proteasome-independent pathway. Proc Natl Acad Sci U S A. 2008;105(29):10119-10124.
- 44. Marijt KA, Blijleven J, Verdegaal EME, Kester MG, et al. Identification of non-mutated neoantigens presented by TAP-deficient tumors. J Exp Med. 2018;215(9):2325-2337.
- 45. Durgeau A, Virk Y, Gros G, et al. Human preprocalcitonin self-antigen generates TAP-dependent and -independent epitopes triggering optimised T-cell responses toward immune-escaped tumours. Nat Commun. 2018;9(1):5097.