The combination of an anti-angiogenic tyrosine kinase inhibitor (TKI) and an anti-PD-1 immunotherapy proved promising, regardless of PD-L1 expression levels, as a first-line therapy for treatment-naïve patients with advanced NSCLC who did not harbor known driver gene mutations.
In a small phase I trial combining the TKI anlotinib and the immunotherapy sintilimab, the overall response rate (ORR) was nearly 73% at a median follow-up of 15.8 months. Median 12-month progression-free survival (PFS) was 71.4% (95% CI, 47.2%-86.0%). Although overall survival (OS) data were immature, its rate was estimated at 95.5% (95% CI, 71.9%-99.3%). Data were presented during OA07: Immuno-biology and Novel Immunotherapeutics from Bench to Bed.
More than one-half of the 22 participating patients experienced treatment-related adverse events of grade 3 or higher.
Lead author Baohui Han, MD, PhD, of Shangai Chest Hospital, said that the combination demonstrated “durable efficacy and good tolerability.”
“Several studies have proved the synergistic efficacy of immunotherapy and anti-angiogenic TKIs; however, evidence to support this chemo-free combination in [patients with] treatment-naïve NSCLC is insufficient,” Han said. “To the best of our knowledge, ours is the first study that assessed a PD-1 inhibitor plus an antiangiogenesis TKI in (the) first-line setting of advanced NSCLC.”
Study Details
His presentation updated efficacy and safety findings from an interim analysis of the trial reported at the World Conference on Lung Cancer in 2019.1 The results from the final analysis include evaluations of a larger array of patient subgroups.
The cohort that received sintilimab and anlotinib—which targets VEGFR, FGFR, PDGFR and c-kit—was one of several trial arms. It included 22 treatment-naïve patients with stage IIIB or IV NSCLC who did not harbor mutations to the EGFR, ALK or ROS1 genes. Patients were enrolled and treated from September 2018 through February 2019. Every 3 weeks, the patients, whose performance status was ECOG 1 or 2 at baseline, received a 200-mg dose of intravenous sintilimab on day 1, as well as 12 mg of oral anlotinib on days 1-14, continuing until disease progression or unacceptable toxicity.
Primary endpoints were ORR and safety, and secondary endpoints included disease control rate (DCR), PFS, and OS.
As of April 30, 2020, median follow-up was 15.8 months (range: 8.3-19.3) and median treatment duration was 14.6 months (range: 3.7-19.3).
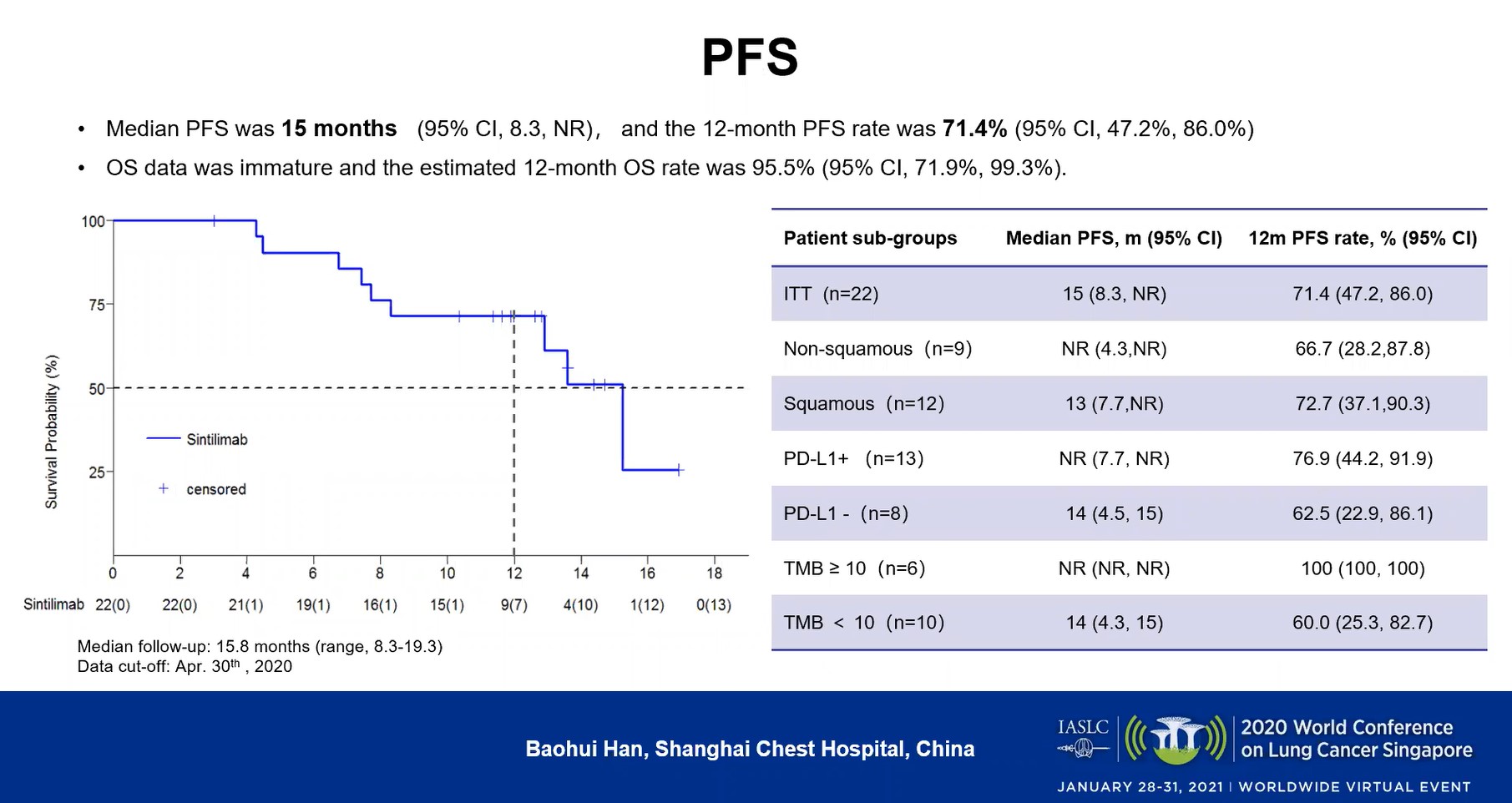
Sixteen of the patients achieved a confirmed partial response, translating to an ORR of 72.7% (49.8%-89.3%). Six patients (27.3%) had stable disease, and the DCR was 100% (84.6%-100%). The median time to response was 1.6 months (95% CI, 1.4-2.9) and the median duration of response had not been reached (95% CI: 3.2 months-NC). The median PFS was 15 months (95% CI, 8.3 months-NR), and the 12-month PFS rate was 71.4% (95% CI, 47.2%-86.0%; Figure).
In considering patient subgroups, the investigators observed that the 9 patients with adenocarcinoma had a higher response rate than the 12 who had squamous-cell disease (88.9% vs 58.3%), but that the two groups had a similar 12-month PFS rate (66.7% vs 72.7%).
They also parsed outcomes based on PD-1 status, finding that the 8 patients with PD-1-negative cancers had a median PFS (95% CI) of 14 months (4.5-15) versus a rate not yet reached for the 13 with PD-1-positive disease (7.7-not reached). The 12-month PFS rates for those two groups (95% CI) were 62.5% (22.9%-86.1%) and 76.9% (44.2%-91.9%), respectively.
The 10 patients with a tumor mutational burden (TMB) under 10 had a median PFS (95% CI) of 14 months (4.3-15) versus a rate not yet reached in the 6 patients with a TMB over 10 (NR-NR); those groups had 12-month PFS rates (95%, CI) of 60% (25.3%-82.7%) versus 100% (100%-100%), respectively.
Han said that all the patients in the trial experienced treatment-related adverse events (TRAEs). Twelve (54.4%) had TRAEs of grade 3 or higher and 1 (4.5%) experienced an immune-related adverse event of grade 3 or higher. Among those that encountered TRAEs of any grade, the most common were hypothyroidism (50%); increased uric acid (40.9%); hand-foot skin reaction (36.4%); hypoalbuminemia (36.4%); and hypertension, increased ALT and increased direct bilirubin (31.8% each).
Five patients required dose reduction of anlotinib and 1 patient discontinued treatment due to toxicity.
“This novel combination has potential efficacy for a broader range of [patients with] NSCLC, regardless of histologic subtype or PD-L1 status,” Dr. Han concluded.
He noted that the combination is being studied in a phase II randomized trial (NCT04124731).
Session discussant Laura Mezquita, MD, PhD, of the Hospital Clinic of Barcelona, praised the team of researchers for their use of a chemotherapy-free strategy, but cautioned that the findings on overall survival are not mature. She added that the patient sample size in the trial was small and suggested that future studies include larger cohorts to assess safety and how efficacy relates to PD-L1 status.
This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 90 days at wclc2020.iaslc.org.
Reference:
- Han B, Chu T, Zhong R, et al. Efficacy and safety of sintilimab with anlotinib as first-line therapy for advanced non-small cell lung cancer. IASLC 2020 World Conference on Lung Cancer Spain; September 7-10, 2019; Abstract JCSE01.11.