NSCLC that harbors more than one DNA damage-response (DDR) gene alteration is more likely to respond to immunotherapy than disease that harbors one or no such mutations.
So concluded investigators who analyzed next-generation sequencing (NGS) of the circulating tumor DNA of patients with advanced NSCLC who had received the PD-L1 inhibitor atezolizumab or the chemotherapy docetaxel.
Wei Nie, MD, PhD, of Shanghai Chest Hospital, and his colleagues conducted their exploratory analysis to seek confirmation of the reported possibility that DDR pathway co-mutations predict checkpoint inhibitor response. The data were presented during the OA07: Immuno-biology and Novel Immunotherapeutics from Bench to Bed session.
While these drugs can spark long-term overall survival for patients with advanced NSCLC, their response rate in that population is about 20% to 30%. “Therefore, identifying the patients who could gain clinical benefit…is a critical unmet need,” Dr. Nie said.
Study Details
The team sought to clarify the function of DDR co-mutations and determine whether they made patients from the populations of the POPLAR and OAK clinical trials more likely to respond to atezolizumab. The researchers selected 853 patients with NSCLC and evaluable NGS results from the cohorts of the trials, both of which tested atezolizumab versus docetaxel in those with previously treated, advanced NSCLC.
Of the selected patients, 429 had taken atezolizumab and 424 docetaxel; 49 had cancer that harbored DDR co-mutations and 804 were co-mutation–negative. The baseline characteristics of patients with and without co-mutations were comparable in terms of age, race, gender, histology, smoking status, lines of treatment, ECOG performance status, and PD-L1 expression.
The investigators used the results of a blood-based FoundationOne NGS assay to identify genetic alterations and blood tumor mutational burden (bTMB) in the patients. They considered nonsense mutations and splice site alterations of DDR genes deleterious, as well as some missense mutations.
The researchers defined co-mutations as deleterious alterations in 2 or more DDR pathways or in 1 such pathway if missense mutation(s) of unknown significance were present in others. They took into account any of 29 DDR genes within 7 DDR pathways.
Table. DNA Damage Response (DDR) Pathways and Their Corresponding Genes
|
DDR Pathway |
DDR Genes |
|
NER |
CUL3 |
|
NHEJ |
MRE11A, PRKDC |
|
HRR |
BRCA1, RAD50, RAD51 |
|
BER |
POLE, POLD1, MUTYH |
|
CPF |
ATM, ATR, CHEK1, CHECK2 |
|
MMR |
MLH1, MSH2, MSH6, PMS2 |
|
FA |
BRIP1, FANCA, FANCL, FANCD2, FANCE, FANCG, FANCC, FANCF, BARD1, BRCA2, PALB2, BLM |
Their analysis compared the objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) of patients with or without co-mutations who were receiving either atezolizumab or docetaxel.
The researchers noted that patients with co-mutations had a significantly longer sum of their tumor diameters (92 [57-144] vs 72 [45-105], p = 0.006) and a higher median bTMB (20 [13.5-29.5] versus 7 [3-15] mutations/Mb, p < 0.001) than patients without co-mutations. Patients with co-mutations also had a significantly higher rate of metastatic sites (3 [2.5-4] vs 3 [2-4], p= 0.026), Dr. Nie said.
Among individuals who took atezolizumab, the investigators observed a significantly higher ORR in those with co-mutations compared with those who were co-mutation-negative (26.7% [n=30] vs 14.8% [n = 365]). Also in those treated with atezolizumab, durable clinical benefit, defined as PFS lasting more than 6 months, was higher in co-mutation-positive patients than in those without co-mutations (56.7% [n = 30] vs 30.6% [n = 399]), Dr. Nie reported.
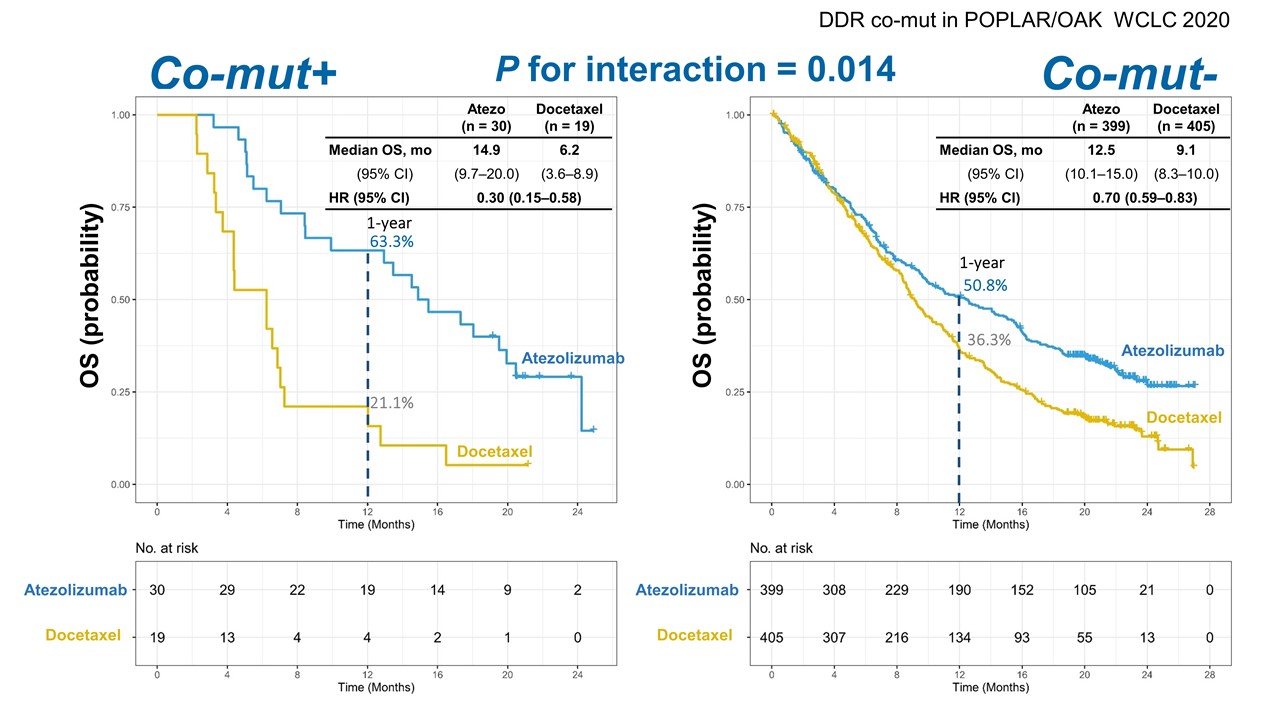
In co-mutation-positive patients who took atezolizumab versus those who received docetaxel, the immunotherapy significantly improved median OS (14.9 months vs 6.2 months; hazard ratio [HR] 0.30; 95% CI: 0.15-0.58) and median PFS (6.9 months vs 3.3 months; HR 0.40; 95% CI: 0.21-0.75), Dr. Nie reported (Figure). In the same population, the median 12-month OS for those receiving atezolizumab was 63.3% compared with 21.1% for individuals receiving docetaxel, and the median 12-month PFS was 28.9% versus 5.3% for those groups, respectively.
Finally, Dr. Nie said, the interaction between co-mutation positivity and treatment was significant for OS (interaction p = 0.014) and PFS (interaction p = 0.010).
“Our study suggested that co-mutation status in the DDR pathway is predictive for the outcome of patients with atezolizumab,” he concluded.
Remaining Questions
He cautioned that the study had limitations: It was exploratory, and just 5.7% of its population was co-mutation-positive, meaning that prospective studies will be needed to confirm its conclusion.
Discussant Myung-Ju Ahn, MD, of Samsung Medical Center in Seoul, praised the study’s large cohort and reliance on blood samples gathered during prospective clinical trials, agreeing that it provided “evidence of the role of co-mutations in the DDR pathway as predictive biomarkers associated with atezolizumab monotherapy.”
Her concerns included the study’s exploratory nature and the fact that the definition of co-mutations in DDR pathways has not been fully validated. Dr. Ahn suggested that blood-based NGS findings in this population should be confirmed with tissue tests. Future studies should consider whether co-mutations predict response to immunotherapies combined with each other or with chemotherapy and should also analyze the predictive value of co-mutations in combination with PD-L1 expression and bTMB, she said.
This session included a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 90 days at wclc2020.iaslc.org.