When patients with advanced metastatic NSCLC initially make their way into our medical oncology clinic, one of the first things we check for is the molecular pathology testing report, in addition to the patient’s PD-L1 score. At the very least, NCCN guidelines recommend testing for EGFR mutations (canonical, uncommon [G719X, S768I, L861Q], and exon 20 insertions), fusions in ALK, ROS1, RET, and NTRK1-3 receptor tyrosine kinase (RTKs). BRAF V600E mutation and MET exon 14 skipping mutation statuses also are important, as are the tumor mutation score and microsatellite status.1 Whenever feasible, broad-based molecular profiling should be considered, as it would allow individualized targeted therapy treatment by identifying rare but targetable molecular alterations such as HER2 exon 20 insertions and NRG1 (neuregulin1) fusion for clinical trials.
NRG1 gene fusion in NSCLC was first described in 2014 by Fernandez-Cuesta and colleagues in a study of invasive mucinous adenocarcinomas of the lung that identified five CD74-NRG1 gene fusions using transcriptome sequencing.2 Multiple fusion partners have been identified in NSCLC but CD74-NRG1 fusion remains the dominant fusion variant.3 Overall, NRG1 fusions are relatively rare.4 The incidence of NRG1 fusions was 0.2% (82 positive cases) in a recent updated large-scale survey of 44,570 cases of solid malignancies profiled by targeted RNA sequencing or whole-transcriptome sequencing.5 Although the vast majority of the NRG1 fusion cases were found in NSCLC compared with other malignancies (54%, 44 of 82), NRG1 NSCLC constituted only 0.3% of all NSCLC samples tested.5NRG1 fusions also were found in renal cell, breast, ovarian, pancreatic, bladder, gallbladder, and colorectal cancers, as well as neuroendocrine tumors and sarcomas.5 Recently, in 2020, Professor Hirokuyi Mano’s group at the National Cancer Center Research Institute in Japan has identified a CD74-NRG2a fusion in NSCLC.6 Following this discovery, we recently performed a pan-tumor survey of NRG2 fusions of ~56,000 solid tumor molecularly profiled by whole transcriptome sequencing and identified 7 NRG2 fusions (0.013%, all a-isoforms of the EGF domain) including two novel fusions,CDH1-NRG2 and F11R-NRG2 , in NSCLC.7
Understanding NRG
NRGs are cellular signaling ligand proteins that all contain the epidermal growth factor (EGF)-like domain and play important roles primarily in the development of the nervous and cardiovascular system through binding to the HER family of receptor tyrosine kinases (RTKs). NRG1 and NRG2 are multiply-spliced members of six distinct but related NRG genes (NRG1, NRG2, NRG3, NRG4, NRG5, NRG6; NRG5 and NRG6 are still known more commonly by other names [Tomoregulin-1, or TMEFF1 (transmembrane protein with EGF like and two follistatin like domains 1) for NRG5; Chondroitin Sulfate Proteoglycan 5 (CSPG5), or Neuroglycan C, or Chicken Acidic Leucine-rich EGF-like domain-containing brain protein (CALEB) for NRG6] where they all contain an EGF-like domain.8 The full EGF-like domain is encoded by two exons where a core EGF-like domain exon alternatively spliced together with one of two unique exons encoding two different amino acid sequences to generate either the a-isoform or b-isoform of the full-length EGF-like domain (NRG1a or NRG1b).9 Importantly, NRG1a and NRG1b have different binding specificities to HER3, its primary receptor HER3.10 Adding to the complexity of NRG1 fusion positive tumor, each tumor seems to harbor different ratio of NRG1a/NRG1b that can be determined by the ratio of the EGF splice site junctional read from next generation RNA sequencing.6
In contrast to other RTK fusions in NSCLC which are constitutively active, NRG1 fusions represent a novel mechanism of generating ligand proximity to the natural occurring wild-type RTKs.11,12 While the genetic rearrangement is structurally and likely mechanistically similar, the biochemical mechanism leading to cancer cell growth is slightly different. NRG1 binds primarily to HER3 through its EGF-like domain which then principally heterodimerizes with HER2 and brings on the activation of downstream signaling pathways promoting oncogenesis.9,10 Nevertheless, HER3 can also form HER3/EGFR, and HER3/HER4 heterodimers. Furthermore, NRG1 can also bind to HER4 albeit at a lower affinity. Indeed, tumor analysis of the NRG1+ NSCLC identified all four members of the HER family members were phosphorylated (not just HER2 and HER3), while only moderate HER4 phosphorylation and no HER2, HER3, or EGFR phosphorylation was seen from NRG2+ NSCLC.6
As NRG1 fusions in NSCLC and other solid malignancies have been reported to have multiple fusion partners beyond CD74 and given the large intronic regions with the NRG1 gene, it is best to detect NRG1 and NRG2 fusion by RNA sequencing preferably by whole transcriptome sequencing.3-7,13 Similar to NTRK fusions, NRG1 fusions and likely NRG2 fusions are examples of molecular alterations in which RNA sequencing is superior compared to next-generation DNA sequencing methods.
Clinical Trials of NRG1 Fusions
Table 1 shows the ongoing studies targeting those with NRG1 fusions. Given the novelty and rarity of NRG2 fusions, no clinical trials are currently targeting NRG2 fusions.6,7,14 The clinical trials targeting NRG1 fusions are tumor-agnostic and involve either pan-HER tyrosine kinase inhibitors (TKIs) or monoclonal antibodies (mAb) against HER3 reflecting the different approaches to target ligand-fusion currently. To date, there are a few published case reports and case series of patients receiving afatinib as targeted therapy for NRG1 fusions and some with success.3,13 Currently, afatinib is being tested in NRG1-mutated solid malignancies in the ASCO-sponsored TAPUR trial (arm 18) (Table 1). Tarloxotinib is the prodrug form of a pan-HER inhibitor that is activated under hypoxic conditions common in tumor tissue and preclinically appears to have activity against NRG1 fusions.15,16
The mAb being evaluated for the treatment of NRG1 fusion positive cancers include zenocutuzumab (MCLA-128) which is a bi-specific HER2/HER3 mAb that blocks the binding of NRG1 to HER3, interrupt the heterodimerization of HER2/HER3 and mediates antibody-dependent cell-mediated cytotoxicity–enhanced (ADCC).17 Preliminary efficacy data of zenocutuzumab against patients harboring ATP1B1–NRG1 (pancreatic) fusions and CD74-NRG1 (NSCLC) have been reported.18,19 Seribantumab (MM-121) is a fully human IgG2 mAb against HER3 and does not mediate ADCC.20 Seribantumab has demonstrated in vitro activity against patient-derived NRG1 fusion xenografts (SLC34A2-NRG1+ NSCLC and CLU-NRG1+ high-grade ovarian carcinoma).21
Given the promiscuities of NRG1 binding and HER3 heterodimerization, it will be interesting to follow if mAb against HER2/3 axis is sufficient to generate durable efficacy. On the other hand, pan-HER TKIs could be overwhelmed eventually by the over-expression of HER family RTKs and/or their ligands.22 Thus, a combination approach may be optimal after efficacy of individual single agents is established.
In summary, the wider use of screening platform including RNA sequencing for NRG1 and NRG2 fusions should lead to further understanding of the complex HER2 pathways including the differential role that fusion partners play in NRG fusions, and appreciation of the subtle binding differences between the NRGa versus NRGb fusion isoforms. Although the cost-effectiveness of detecting such a rare fusion remains a debate at the countrywide population level, for that one patient identified with the sensitizing NRG1 (or NRG2) fusion, it allows for clinical trials and off-label use of existing therapy, expanding our horizons in understanding the oncogenesis of ligand-fusion alterations.
Table 1. Ongoing Studies for Patients With NRG1 Fusion–Positive Malignancies
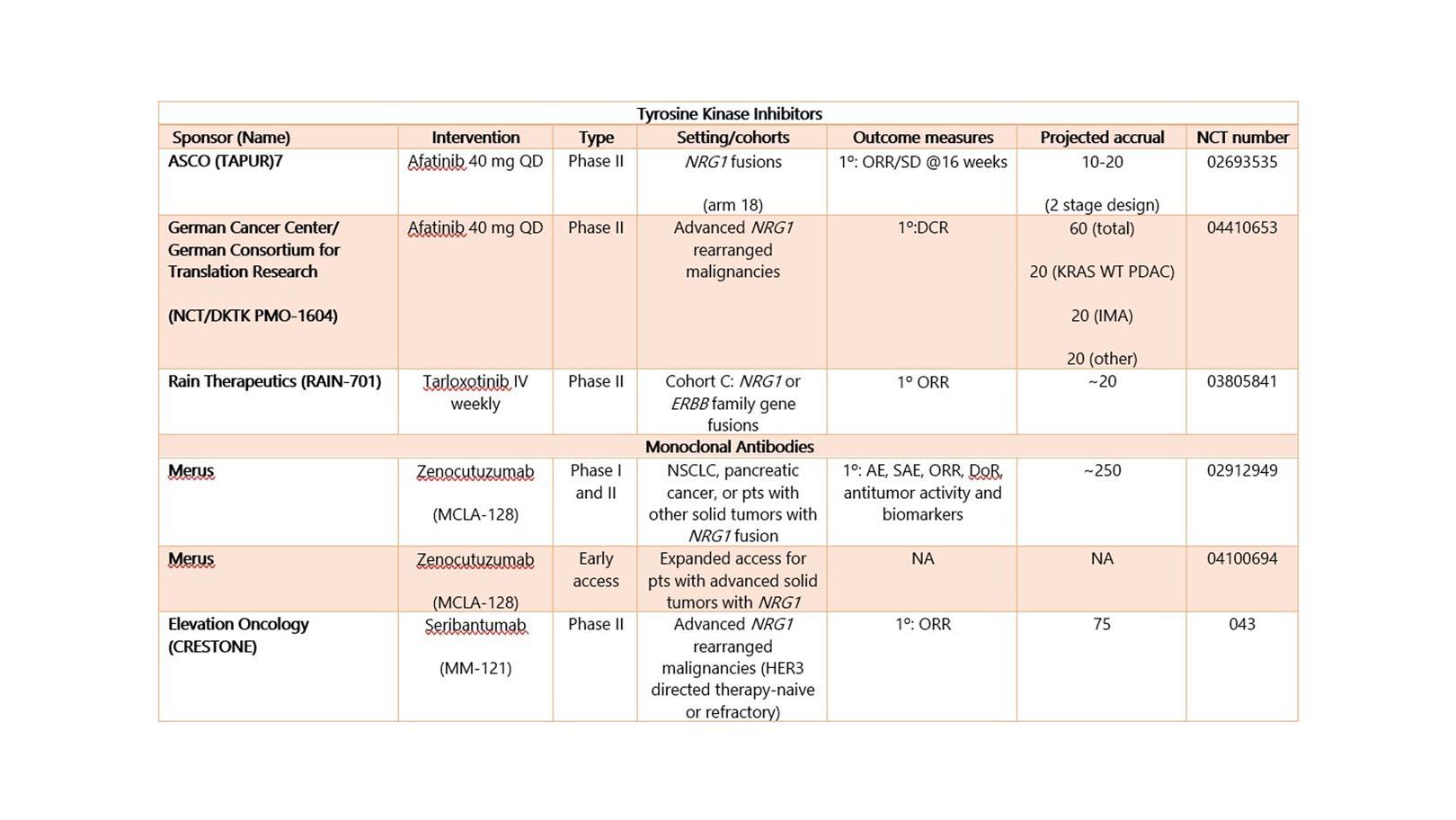
AE, adverse event; ASCO, American Society of Clinical Oncology; DCR, disease control rate; DoR, duration of response; IMA, invasive mucinous adenocarcinoma; NA, not available; ORR, overall response rate; OS, overall survival; PDAC, pancreatic ductal adenocarcinoma; PFS, progression-free survival; SAE, severe adverse events; SD, stable disease; TAPUR, Targeted Agent and Profiling Utilization Registry; WT, wildtype.
References:
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. Version 6.2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Published June 15, 2020. Accessed August 15, 2020.
- Fernandez-Cuesta L, Plenker D, Osada H, et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014; 4(4):415-422.
- Nagasaka M, Ou SI. Neuregulin 1 Fusion-Positive NSCLC. J Thorac Oncol. 2019; 14(8): 1354-1359.
- Jonna S, Feldman RA, Swensen J, et al. Detection of NRG1 fusions in solid tumors. Clin Cancer Res. 2019;25(16):4966-4972.
- Jonna S, Feldman R, Ou SHI, et al. Characterization of NRG1 gene fusion events in solid tumors. J Clin Oncol. 2020;38(15)_suppl (May 20, 2020) 3113-3113.
- Kohsaka S, Hayashi T, Nagano M, et al. Identification of Novel CD74-NRG2α Fusion from comprehensive Profiling of Lung Adenocarcinoma in Japanese Never or Light Smokers. J Thorac Oncol. 2020;15(6):948-961.
- Ou SI, Xiu J, Nagasaka M, et al. Identification of novel CDH1-NRG2 and F11R-NRG2 fusions in NSCLC plus additional novel NRG2a fusions in other solid tumors by whole transcriptome sequencing. JTO-Clin Research Reports 2020 in press
- Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2015; 83(1):27-49.
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003; 284(1):14-30.
- Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett. 1999;447:227-31.
- Fernandez-Cuesta L, Thomas RK. Molecular pathways: targeting NRG1 fusions in lung cancer. Clin Cancer Res. 2015;21(9):1989-1994.
- Dimou A, Camidge DR. Detection of NRG1 fusions in solid tumors: Rare gold? Clin Cancer Res. 2019 ;25:4865-4867.
- Drilon A, Somwar R, Mangatt BP, et al. Response to ERBB3-directed targeted therapy in NRG1-rearranged cancers. Cancer Discov. 2018;8(6):686-695.
- Nagasaka M, Ou SI. Is NRG2α fusion a “doppelgänger” to NRG1α/β fusions in oncology? J Thorac Oncol. 2020 Jun;15(6):878-880.
- Tarloxotinib. Rain Therapeutics, Inc. rainthera.com/tarloxotinib-as-a-novel-therapeutic-strategy-for-oncogenic-alterations-across-the-erbb-family-of-receptors. Accessed October 22, 2020.
- Tirunagaru VG, Estrada-Bernal A, Yu H, et al. Tarloxotinib exhibits potent activity in NRG1 fusion and rearranged cancers (Abstract 2202). Paper presented at: AACR Annual Meeting 2019. March 29-April 3, 2019; Atlanta, Georgia.
- Geuijen CAW, De Nardis C, Maussang D, et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2-guided ligand blockade. Cancer Cell. 2018 May 14;33(5):922-936.
- Bispecific antibody MCLA-128 shows clinical activity in patients with solid tumors harboring NRG1 gene fusions. American Association for Cancer Research. aacr.org/about-the-aacr/newsroom/news-releases/bispecific-antibody-mcla-128-shows-clinical-activity-in-patients-with-solid-tumors-harboring-nrg1-gene-fusions. Published October 27, 2019. Accessed October 22, 2020.
- MCLA-128 fights NRG1 fusion-positive cancers. Cancer Discov. 2019 Dec;9(12):1636.
- Schoeberl B, Kudla A, Masson K, et al. Systems biology driving drug development: from design to the clinical testing of the anti-ErbB3 antibody seribantumab (MM-121). NPJ Syst Biol Appl. 2017 Jan 5;3:16034.
- Odinstov I, et al. The anti-HER3 monoclonal antibody seribantumab effectively inhibits growth of patient-derived and isogenic cell line and xenograft models with NRG1 rearrangements. https://elevationoncology.com/ena-2020/assessed December 15, 2020
- Jacob W, James I, Hasmann M, Weisser M. Clinical development of HER3-targeting monoclonal antibodies: perils and progress. Cancer Treat Rev. 2018;68:111-123.






