Kirsten rat sarcoma (KRAS) has long been known as one of the most mutated clonal oncogenic drivers in non-small cell lung cancer (NSCLC). However, despite its wide prevalence and pivotal role in driving tumor growth, it was also long considered undruggable because of its high GTP (guanosine triphosphate) affinity and relatively smooth surface—difficult challenges to overcome when designing drugs.1 Inhibition of downstream signaling or membrane localization of KRAS had been generally unsuccessful.2,3,45
Nevertheless, novel therapeutic approaches, including vaccine and cell therapy-based approaches, have recently emerged and provide new hope for NSCLC patients with KRAS mutations. This article will focus on the development of small molecule inhibitors that target KRAS mutations in NSCLC.
The most common form of KRAS dysregulation in NSCLC arises from amino acid changes mediated by point mutations in codons 12 and 13 in exon 2 of the KRAS gene, resulting in constitutive activation.6 Moreover, co-occurring genomic alterations, such as TP53, STK11, KEAP1, etc., may affect prognosis and treatment outcomes.8 Modern genomic profiling identified that about a third of lung adenocarcinomas harbor a KRAS mutation with G12C as the most common subtype (30% – 40%), which also became the first druggable target9.

Monotherapy Targeting KRASG12C Subvariant
The seminal work from the Shokat group paved the way for the development of direct G12C inhibitors.10 A new pocket, beneath the effector binding switch-II region, was identified, which led to the identification of a G12C-specific inhibitor that covalently locks KRAS in its inactive GDP-bound state, thus disrupting downstream oncogenic signaling. Thereafter, novel compounds have been developed and tested in clinical trials, leading to a historic approval of sotorasib in 2021 by the U.S. Food and Drug Administration.
Sotorasib is the first-in-class KRAS mutation-specific covalent inhibitor that irreversibly and selectively binds to the switch II pocket of KRASG12C in its inactive conformation (OFF). In the phase II portion of the single-arm CodeBreak 100, 960mg daily of sotorasib was evaluated in 126 enrolled patients with NSCLC who had received prior platinum-based chemotherapy and/or anti-PD-(L)1 treatment.11 The study reported an objective response rate (ORR) of 37.1%, disease control rate (DCR) of 80.6%, median progression-free survival (PFS) of 6.8 months, and median overall survival (OS) of 12.5 months. Grade 3 treatment-related adverse events (TRAEs) were observed in 19.8% patients, however there were no grade 5 TRAEs and treatment discontinuation rate due to adverse events was only 7%.

These favorable results compared to historic estimates with docetaxel-based therapy led to its accelerated approval by US FDA in May 2021. A longer follow-up at 2 years (pooled phase I and II cohorts receiving 960mg daily dose) showed a 2-year OS rate at 32.5%. Importantly, there was no delayed onset of adverse events.12 Notable is that all subgroups of patients derived benefit, particularly those with low PD-L1 expression or STK11 co-mutation who derive less benefit from immunotherapy.
To further confirm the efficacy of sotorasib, CodeBreak 200 directly compared sotorasib to docetaxel in previously treated NSCLC. The study met its primary endpoint of PFS improvement (median 5.6 vs. 4.5 months; HR 0.66, 95% CI 0.51 – 0.86) as well as other secondary endpoints such as response rate, duration of benefit, and quality-of-life improvement.13 However, the lack of OS benefit is disappointing. We should keep in mind that the study was amended to reduce sample size and was not powered for OS. Additionally, cross-over was allowed in this study and 34% of patients in docetaxel arm received subsequent KRASG12C inhibition. In the era of first-generation EGFR or ALK tyrosine kinase inhibitors (TKIs), none of them showed OS benefit compared to chemotherapy due to cross-over as well, despite an absolute PFS benefit that was larger with these EGFR/ALK inhibitors.14,15,16
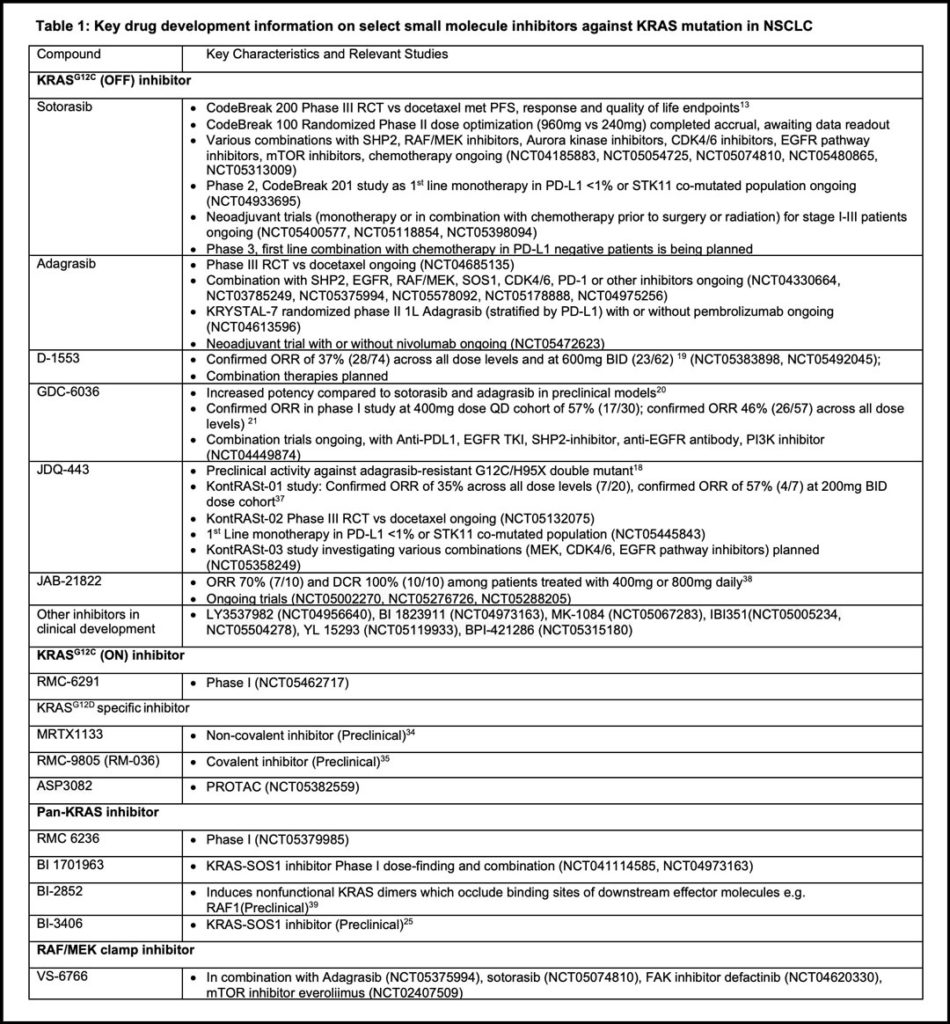
Adagrasib is another KRASG12C (OFF) inhibitor that has mature clinical data reported to date. The phase 1-1b part of KRYSTAL-1 reported a similar ORR of 42.9% and median PFS at 6.5 months among 116 previously treated patients. Intracranial confirmed objective response was 33.3% in a subset of patients with previously treated central nervous system metastases. Grade 3 or higher TRAEs occurred in 44.8% of patients (including two grade 5 events) requiring dose reduction or interruption in most patients, with a drug discontinuation rate of 6.9%.17 As seen with sotorasib, response rates were similar in STK11 co-mutant and low PD-L1 subgroups. Table 1 lists other relevant KRASG12C (OFF) inhibitors.18,19,20,21
Resistance to Direct G12C Inhibitors
KRAS is known to be often accompanied by other co-occurring mutations. Studies of limited sample sizes have inconsistently shown the adverse impact of such co-mutations, e.g. in KEAP1, on primary resistance. 17 With regards to acquired resistance, unlike the role of gatekeeper EGFR T790M mutation as the major secondary resistance mechanism to first generation EGFR TKIs, the putative resistance mechanism to KRASG12C (OFF) inhibitors are rather heterogeneous. Preclinical and clinical studies with paired biopsies have shown that the mechanisms largely fall into the following categories: 1) on-target resistance; 2) off-target bypass resistance 3) histological transformation 4) tumor microenvironment.22 What makes overcoming resistance more challenging is that up to 41% of tumors had more than one concurrent potential resistance mechanism.22
Beyond KRASG12C Monotherapy
Due to the relatively modest efficacy of direct G12C (OFF) inhibitors and the development of acquired resistance, multiple combinations with other therapy are being actively explored in the metastatic or even neoadjuvant setting (Table 1).
Preclinical studies have identified that an upstream RTK (receptor tyrosine kinase) regulator, SHP2, may mediate escape from KRASG12C inhibition and coadministration of SHP2 inhibitors can augment the anti-tumor efficacy of G12C inhibitors in mouse models.23 A combination of sotorasib and the SHP2 inhibitor RMC-4630 in 21 patients (11 with NSCLC) was recently presented with an objective response rate of 50% and 27% in G12C inhibitor–naïve and treated patients, respectively.24 Targeting small molecule SOS1 is also being actively tested. SOS1 binds inactive GDP-bound KRAS, facilitating the exchange of GDP to GTP.25 It is hypothesized that by inhibiting SOS1, all forms of KRAS may be inactivated. Because of the importance of MAPK pathway downstream of RAS, a novel RAF/MEK clamp VS-6766 with a reduction of feedback-driven pathway is being tested. Early phase clinical trials show promising antitumor efficacy across a spectrum of KRAS mutant tumors. Trials are ongoing among KRAS mutated NSCLC patients.26,27
Given that immune checkpoint inhibitors have demonstrated survival advantage when incorporated into first-line regimens for advanced disease and that preclinical data show synergism between KRASG12C and immune checkpoint inhibition, there is a high level of interest to explore the safety and efficacy of such combinations. However, recent data from CodeBreak 100/101 combining sotorasib with pembrolizumab or atezolizumab concurrently or sequentially28 reported high rates of grade 3+ hepatotoxicity with half of patients discontinuing treatment due to toxicity. Although lead-in sotorasib dosing resulted in less adverse events, the proportion of grade 3+ toxicity was still as high as 53% without evidence of improved efficacy. Thus, plans are underway to combine sotorasib with chemotherapy only as first-line treatment for patients with PD-L1 < 1%.29
In comparison, the initial report from KRYSTAL-7 demonstrates potential increased response rates although longer follow-up is needed to assess the safety profile as hepatotoxicity signals generally occur several weeks after the typical dose-limiting toxicity observation period.30 Given the cumulative experience with encountering significant toxicities in combining various small molecule inhibitors (EGFR, BRAF/MEK, ALK) with anti-PD(L)1 therapy in general with few exceptions,31 optimal sequencing of KRASG12C inhibitors with anti-PD(L)1 directed therapy needs to be defined, and whether there will be agent that can be safely combined over a longer period of time remains to be demonstrated.
Other Small Molecule Approaches Targeting KRAS
Table 1 summarizes other small molecule approaches targeting KRAS. Contrary to targeting the GDP-bound OFF form used by sotorasib and most other agents in development, RMC-6291 is a novel molecule that can bind to the active state ON form of KRASG12C by acting as a molecular glue with cyclophilin A, blocking downstream RAS effector signaling. Based on promising preclinical data, a clinical trial is currently ongoing.
RMC 6236, a multiple RAS (ON) inhibitor using the same tricomplex approach described above also exhibited preclinical activity across various KRAS mutants32. Safety and efficacy data of this class of agents are awaited.
KRASG12D/V are the next two most common subtypes in LUAD, accounting for about 15% individually33. Antitumor activity of novel direct noncovalent G12D inhibitors such as MRTX1133 or covalent inhibitors such as RMC-9805 (another cyclophilin A-based molecular glue) have been reported in preclinical models34,35. Plans for clinical testing are underway.
Proteolysis targeting chimeras (PROTACs) are a novel class of drug, which are bifunctional molecules that simultaneously bind a protein of interest and an E3 ligase. The latter then ubiquitinates and degrades the protein of interest36. Various agents are in preclinical and clinical development.
Conclusion
Almost 40 years passed between the discovery of KRAS oncogenic mutations and the approval of a clinically viable therapeutic agent for NSCLC. While this targeted therapy is welcome, unanswered questions remain.
- How can we move direct G12C inhibitors beyond second line?
- How can we prevent or treat resistance?
- How can we improve efficacy as monotherapy and with combination strategies, including optimization of CNS activity?
- How can we identify patients best suited for particular combination strategies to improve therapeutic index?
- How can we target other KRAS subvariants?
- How can we successfully develop a pan-KRAS inhibitor with sufficient therapeutic index?
The future is bright with stakeholders united in the common goal to improve the lives of NSCLC patients with KRAS mutations.
References
- 1. Nissley DV, McCormick F. RAS at 40: Update from the RAS Initiative. Cancer Discov 2022;12:895-898.
- 2. Goldman JW, Mazieres J, Barlesi F, et al. A Randomized Phase III Study of Abemaciclib Versus Erlotinib in Patients with Stage IV Non-small Cell Lung Cancer With a Detectable KRAS Mutation Who Failed Prior Platinum-Based Therapy: JUNIPER. Front Oncol 2020;10:578756.
- 3. Janne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib Plus Docetaxel Compared With Docetaxel Alone and Progression-Free Survival in Patients With KRAS-Mutant Advanced Non-Small Cell Lung Cancer: The SELECT-1 Randomized Clinical Trial. JAMA 2017;317:1844-1853.
- 4. Riely GJ, Johnson ML, Medina C, et al. A phase II trial of Salirasib in patients with lung adenocarcinomas with KRAS mutations. J Thorac Oncol 2011;6:1435-1437.
- 5. Adjei AA, Mauer A, Bruzek L, et al. Phase II study of the farnesyl transferase inhibitor R115777 in patients with advanced non-small-cell lung cancer. J Clin Oncol 2003;21:1760-1766.
- 6. Reck M, Carbone DP, Garassino M, et al. Targeting KRAS in non-small-cell lung cancer: recent progress and new approaches. Ann Oncol 2021;32:1101-1110.
- 7. Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res 2008;14:5731-5734.
- 8. Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov 2018;8:822-835.
- 9. Judd J, Abdel Karim N, Khan H, et al. Characterization of KRAS Mutation Subtypes in Non-small Cell Lung Cancer. Mol Cancer Ther 2021;20:2577-2584.
- 10. Ostrem JM, Peters U, Sos ML, et al. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 2013;503:548-551.
- 11. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-2381.
- 12. Dy GK, Govindan R, Velcheti V, et al. Abstract CT008: Long-term outcomes with sotorasib in pretreated KRASp.G12C-mutated NSCLC: 2-year analysis of CodeBreaK100. Cancer Research 2022;82:CT008-CT008.
- 13. Johnson ML, de Langen AJ, Waterhouse DM, et al. LBA10 Sotorasib versus docetaxel for previously treated non-small cell lung cancer with KRAS G12C mutation: CodeBreaK 200 phase III study. Annals of Oncology 2022;33:S1417-S1418.
- 14. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-246.
- 15. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-2874.
- 16. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-2394.
- 17. Janne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. N Engl J Med 2022;387:120-131.
- 18. Weiss A, Lorthiois E, Barys L, et al. Discovery, Preclinical Characterization, and Early Clinical Activity of JDQ443, a Structurally Novel, Potent, and Selective Covalent Oral Inhibitor of KRASG12C. Cancer Discov 2022;12:1500-1517.
- 19. Lu S, Jian H, Zhang Y, et al. OA03.07 Safety and Efficacy of D-1553 in Patients with KRAS G12C Mutated Non-Small Cell Lung Cancer: A Phase 1 Trial. Journal of Thoracic Oncology 2022;17:S11.
- 20. Purkey H. Abstract ND11: Discovery of GDC-6036, a clinical stage treatment for KRAS G12C-positive cancers. Cancer Research 2022;82:ND11-ND11.
- 21. Sacher A, Patel MR, Miller WH, Jr., et al. OA03.04 Phase I A Study to Evaluate GDC-6036 Monotherapy in Patients with Non-small Cell Lung Cancer (NSCLC) with KRAS G12C Mutation. Journal of Thoracic Oncology 2022;17:S8-S9.
- 22. Awad MM, Liu S, Rybkin, II, et al. Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N Engl J Med 2021;384:2382-2393.
- 23. Nichols RJ, Haderk F, Stahlhut C, et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat Cell Biol 2018;20:1064-1073.
- 24. Falchook G, Li BT, Marrone KA, et al. OA03.03 Sotorasib in Combination with RMC-4630, a SHP2 Inhibitor, in KRAS p.G12C-Mutated NSCLC and Other Solid Tumors. Journal of Thoracic Oncology 2022;17:S8.
- 25. Hofmann MH, Gmachl M, Ramharter J, et al. BI-3406, a Potent and Selective SOS1-KRAS Interaction Inhibitor, Is Effective in KRAS-Driven Cancers through Combined MEK Inhibition. Cancer Discov 2021;11:142-157.
- 26. Guo C, Chénard-Poirier M, Roda D, et al. Intermittent schedules of the oral RAF-MEK inhibitor CH5126766/VS-6766 in patients with RAS/RAF-mutant solid tumours and multiple myeloma: a single-centre, open-label, phase 1 dose-escalation and basket dose-expansion study. Lancet Oncol 2020;21:1478-1488.
- 27. Minchom AR, Perez VS, Morton C, et al. Phase I trial of the RAF/MEK clamp VS-6766 in combination with everolimus using an intermittent schedule with expansion in NSCLC across multiple KRAS variants. Journal of Clinical Oncology 2022;40:9018-9018.
- 28. Li BT, Falchook GS, Durm GA, et al. OA03.06 CodeBreaK 100/101: First Report of Safety/Efficacy of Sotorasib in Combination with Pembrolizumab or Atezolizumab in Advanced KRAS p.G12C NSCLC. Journal of Thoracic Oncology 2022;17:S10-S11.
- 29. Amgen ESMO 2022 Investor Event. Available at https://investors.amgen.com/static-files/22a84c17-86fd-43d3-932a-189c4ec7005b. Accessed October 21, 2022
- 30. MRTX Corporate Presentation. Available at https://s27.q4cdn.com/140416303/files/doc_presentation/2022/09/Mirati_Corporate-Presentation_12Sept2022.pdf. October 21, 2022.
- 31. Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020;395:1835-1844.
- 32.. Koltun ES, Rice MA, Gustafson WC, et al. Abstract 3597: Direct targeting of KRASG12X mutant cancers with RMC-6236, a first-in-class, RAS-selective, orally bioavailable, tri-complex RASMULTI(ON) inhibitor. Cancer Research 2022;82:3597-3597.
- 33. Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 2015;5:860-877.
- 34. Wang X, Allen S, Blake JF, et al. Identification of MRTX1133, a Noncovalent, Potent, and Selective KRAS(G12D) Inhibitor. J Med Chem 2022;65:3123-3133.
- 35. Knox JE, Jiang J, Burnett GL, et al. Abstract 3596: RM-036, a first-in-class, orally-bioavailable, Tri-Complex covalent KRASG12D(ON) inhibitor, drives profound anti-tumor activity in KRASG12D mutant tumor models. Cancer Research 2022;82:3596-3596.
- 36. Bekes M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov 2022;21:181-200.
- 37. Tan DS, Shimizu T, Solomon B, et al. Abstract CT033: KontRASt-01: A phase Ib/II, dose-escalation study of JDQ443 in patients (pts) with advanced, KRAS G12C-mutated solid tumors. Cancer Research 2022;82:CT033-CT033.
- 38. Li J, Zhao J, Cao B, et al. A phase I/II study of first-in-human trial of JAB-21822 (KRAS G12C inhibitor) in advanced solid tumors. Journal of Clinical Oncology 2022;40:3089-3089.