Editor’s Note: For a patient perspective on the first-line use of chemotherapy for non-small cell lung cancer patients with EGFR mutations, read an essay by EGFResisters Co-Founder Ivy Elkins.

The publishing of data from the FLAURA21 and MARIPOSA2 trials in the fall of 2023 spurred much discussion and debate about the use of first-line chemotherapy in non-small cell lung cancer (NSCLC) patients with EGFR mutations.
To clarify the role of first-line chemotherapy in this population, Suresh S. Ramalingam, MD, and Joshua K. Sabari, MD, debated the pros and cons during the 2024 Targeted Therapies of Lung Cancer meeting, which took place February 21-24 in Santa Monica, California.
Dr. Ramalingam, Executive Director of Winship Cancer Institute of Emory University, Atlanta, argued first-line therapy for all EGFR patients should be chemotherapy plus a TKI. He said there are three key points to consider when reviewing the evidence: improved progression-free survival (PFS), central nervous system (CNS) activity, and good tolerability.
“Let’s look at how osimertinib became first-line therapy in the first place,” he said. “Compared to previous generations of treatments, there was improvement in PFS, improvement in CNS activity, and good tolerance. I would say the answer is very clear that chemo plus osimertinib should be standard of care [for the same reasons].”
Dr. Ramalingam supported his position by reviewing data from FLAURA2, which showed a 10-month improvement in median PFS when patients received chemotherapy plus osimertinib compared to osimertinib alone. Additionally, patients with brain metastasis also had about a 10-month improvement in median PFS,
“The other point to keep in mind is when patients get first-line osimertinib—these are data from the FLAURA study—only about 65% of the patients went on to receive a subsequent therapy after first-line osimertinib. There’s a one out of three chance that your first-line therapy is the last therapy that the patient will see. So you want to maximize the ability to achieve durable disease control, and that’s another reason to think about using chemo plus osimertinib in the frontline setting.”

Dr. Ramalingam also addressed the heterogeneity of EGFR-mutated lung cancers.
“Reports show that EGFR-mutated lung cancers are not a monolithic entity,” he said. “There’s clonal heterogeneity. There are co-mutations. Therefore, single agent therapy may not be able to achieve what combination therapy can achieve… We also know that when you give chemo plus osimertinib, you stand a better chance of avoiding the development or emergence of drug-tolerant persister cells. So that’s yet another reason why chemo plus osimertinib in this patient population may be beneficial.”
However, Dr. Sabari, Assistant Professor in the Department of Medicine at New York University Grossman School of Medicine, New York, said first-line TKI monotherapy remains the preferred therapy for many EGFR-positive patients.
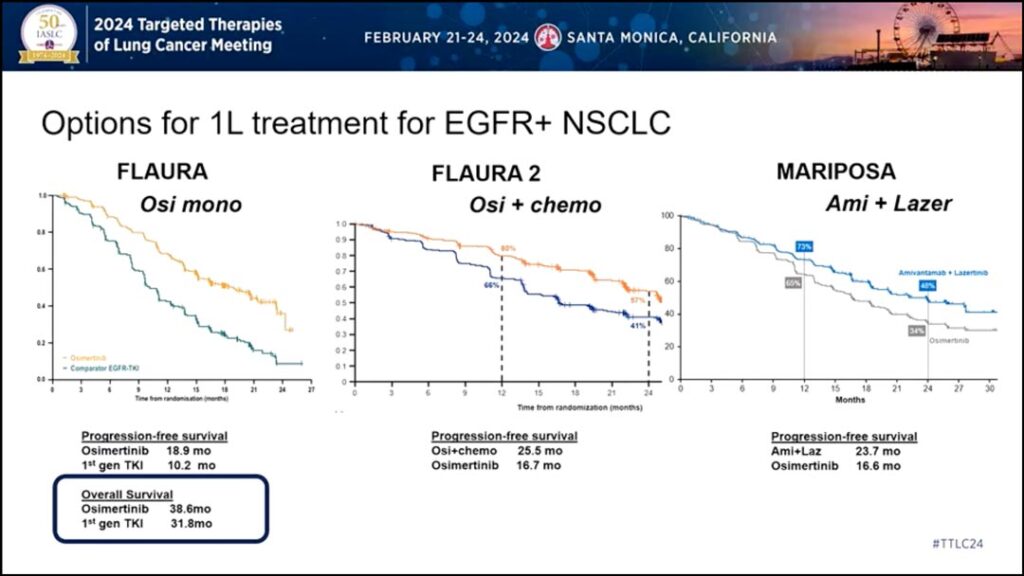
First, Dr. Sabari said the overall survival (OS) data from FLAURA2 and MARIPOSA remains immature (Fig. 1).
“We’ve talked about these three trials: FLAURA, FLAURA2, MARIPOSA,” he said. “Clearly, we’re improving progression free survival, but to my knowledge the only trial to show overall survival here is the FLAURA trial [with osimertinib monotherapy] with 38.6 months. Are these other trials going to meet that? We don’t have that data yet…
“To combine therapies, there needs to be a clear improvement in progression free survival that is greater than the sum of using both therapies sequentially… But more importantly, it must improve overall survival. We know we are improving progression free survival, and maybe we’re eradicating persister sub clones, but does that lead to an actual overall survival benefit?”
Dr. Sabari also said the addition of chemotherapy to the frontline comes with costs.
“What are the costs?” he asked. “There are many… The toxicities here are not just financial. There are toxicities to our patients. Looking at amivantamab plus lazertinib in the MARIPOSA trial, in the frontline we see a threefold increase in venous thromboembolism (VTE), increased rate of infusion-related reactions, increased rate of rash and dermatitis. I think these are important for our patients.”
Given the significant heterogeneity in the EGFR population, Dr. Sabari said adding chemotherapy to the frontline may lead to unnecessarily over-treating some patients.
“Chemotherapy is actually quite toxic,” he said. “One of the most toxic medications available. When you look at carboplatin-pemetrexed… we see two- to threefold increase in leukopenia, thrombocytopenia, and anemia. These are real toxicities for our patients.”
Instead of giving all patients chemotherapy plus TKI upfront, Dr. Sabari said monotherapy should be standard for most with the option to add chemotherapy for some patients who are likely to do poorly on monotherapy.
Patient selection matters, he said, and he asked the audience to image two patients.
“Imagine a 76-year-old woman with an exon 19 deletion, low burden of disease, no CNS metastasis, slow growing,” Dr. Sabari said. “You check CT DNA 3 to 4 weeks out, and it’s negative. Maybe that’s a patient who may benefit from osimertinib alone. Then look at our other patient… a 52-year-old patient with an uncommon EGFR mutation, high burden of disease, and progression within 4 months of therapy. Do we treat these patients the same way?”
He referenced an ongoing study3 lead by thoracic oncologist Helena Yu, MD, of Memorial Sloan Kettering Cancer Center, New York, that will begin all patients with 3 weeks of osimertinib and then randomize them to either continue monotherapy or add chemotherapy based on ctDNA results.
“To me, that’s a more delicate approach,” he said. “I strongly believe that osimertinib monotherapy should be the default standard for most. And again, there’s an opt-in strategy for high-risk patients predicted to do poorly with monotherapy. Those are patients where we should consider osimertinib plus chemo or, when it’s approved, amivantamab plus lazertinib.”
References
- 1. Planchard D, Jänne PA, Cheng Y, et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med. 2023;389(21):1935-1948. doi:10.1056/NEJMoa2306434
- 2. Cho BC, Felip E, Hayashi H, et al. MARIPOSA: phase 3 study of first-line amivantamab + lazertinib versus osimertinib in EGFR-mutant non-small-cell lung cancer. Future Oncol. 2022;18(6):639-647. doi:10.2217/fon-2021-0923
- 3. NCT04410796, PI Yu