Adagrasib, an oral, covalent inhibitor of KRASG12C, showed durable response against KRASG12C-mutated advanced or metastatic non-small cell lung cancer (NSCLC). After a median follow-up pf 26.9 months, the phase 1/2 KRYSTAL-1 trial showed median overall survival (OS) of 14.1 months and median progression-free survival (PFS) of 6.9 months. The agent was granted accelerated approval by the Food and Drug Administration in December 2022 based on 1-year results of the phase 2 trial.

“The data demonstrate compelling results, providing hope for patients with this mutation,” said Shirish M. Gadgeel, MD, Professor and Head of Hematology/Oncology and Associate Director for Patient Experience and Clinical Care at the Henry Ford Cancer Institute. “Exploratory analyses indicated that adagrasib provided promising clinical benefit for a broad group of patients, including those presenting with baseline CNS metastases and co-mutation including DEAP1, STK11, and TP53.”
Dr. Gadgeel presented “KRYSTAL-1: Two-Year Follow-Up of Adagrasib (MRTX849) Monotherapy in Patients with Advanced/Metastatic KRASG12C-Mutated NSCLC” during a mini-oral session on Sunday, September 10. The session, Targeted Therapy: KRAS and Beyond, is available on-demand for registered WCLC attendees until December 31.
KRYSTAL-1 enrolled a total of 132 patients—16 in a phase I/1b dose escalation and expansion trial and the remaining 116 in a phase 2 monotherapy trial. The primary endpoint was overall response rate per blinded independent clinical review. Secondary endpoints included duration of response, progression-free survival, 1-year survival, overall survival, and safety. Exploratory endpoints looked at clinical activity in patients with CNS metastases or with co-mutations at baseline.
All patients had unresectable or metastatic NSCLC with documented KRASG12C mutations.
Objective responses were seen in 43% of patients, Dr. Gadgeel reported a disease control rate of 80%. The median duration of response was 12.4 months.
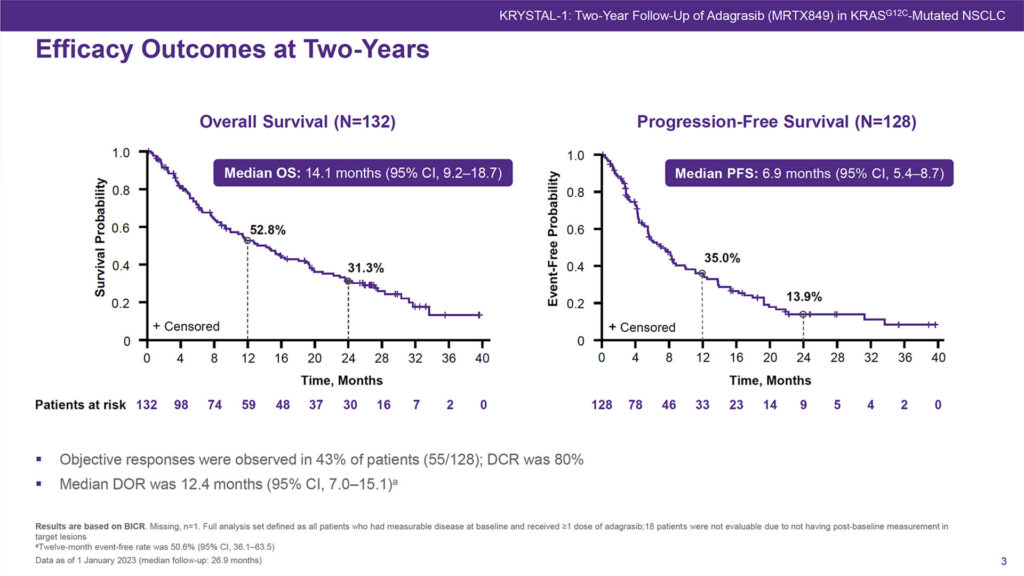
More than half of patients, 52.8%, were alive at 12 months and 31.3% at 24 months. Just over a third of patients, 35.0%, remained progression-free at 12 months and 13.9% were progression-free at 24 months.
“Efficacy was observed in patients with co-mutations and with CNS metastases,” he said. Median OS in patients with CNS metastases at baseline was 14.7 months and median PFS was 6.9 months. For patients with co-mutations at baseline, median overall survival ranged from 18.7 months for TP53 to 5.7 months for KEAP1 with median PFS from 8.7 months for TP53 to 4.1 months for KEAP1.
Nearly all patients in the trial had dose reductions or modifications, including all 55 of the responders who remained alive at 2 years, Dr. Gadgeel said. But dose reductions did not seem to impact clinical activity. For all patients with dose modifications, 1-year OS was 53.3%, 2-year OS was 32.1%.
There were no new safety signals seen with longer-term treatment, he added, and few new treatment-related adverse events after the first year of treatment. The most common adverse events include diarrhea, nausea/vomiting, fatigue, and increased serum creatinine. None of the patients who received immunotherapy within 30 days of starting adagrasib had grade ≥3 hepatoxicity and only one patient discontinued treatment due to grade 3 hepatoxicity.
A confirmatory phase 3 global study, KRYSTAL-12, comparing adagrasib versus docetaxel, in previously treated patients with KRASG12C-mutated NSCLC is under way in Asia, Australia, Europe, and North America. The trial is expected to be completed by late 2025.




